

46550-G1
The Development of Mild and Operationally Simple Direct Methods for the Synthesis of beta-Dicarbonyl Compounds
Soft enolization has been shown to provide an exceptionally mild and operationally simple approach to direct carbon-carbon bond formation applicable to 1,3-diketone and b-keto thioester synthesis.
Crossed-Claisen condensation. We anticipated that the use of a thioester as the
enolate precursor, in combination with an acid chloride or N-acylbenzotriazole as an acyl donor would
enable chemoselective enolization The
usefulness of the present method in preparing b-keto thioesters, along with the strategic advantage presented in their
synthetic equivalence to b-keto
acids, was demonstrated by a concise total synthesis of LY294002 (62) (Figure 7), a potent and specific
inhibitor of PI3-K.
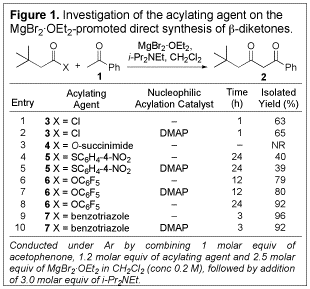 1,3-Diketone synthesis. To explore the use of
soft enolization in 1,3-diketone synthesis, acetophenone was combined with a
variety of known acylating agents both with and without added DMAP as a
nucleophilic acylation catalyst. The results are summarized in Figure 1.
Addition of DMAP was uniformly of no benefit with regard to either the time required
for the reaction or the yield produced (entries 2, 5, 7, and 10). O-Succinimide ester 4 failed to react altogether, and while
thioester 5 did produce
the desired product, yields were lower than for the corresponding acid chloride
(3). O-Pfp ester 6 proved to be a suitable acylating agent,
giving 79% yield of the b-diketone within 12 h
and 92% within 24 h. Even better yields and shorter reaction times resulted
from the use of N-acylbenzotriazole
7. Thus, both O-Pfp esters and N-acylbenzotriazoles were investigated in our
subsequent studies.
1,3-Diketone synthesis. To explore the use of
soft enolization in 1,3-diketone synthesis, acetophenone was combined with a
variety of known acylating agents both with and without added DMAP as a
nucleophilic acylation catalyst. The results are summarized in Figure 1.
Addition of DMAP was uniformly of no benefit with regard to either the time required
for the reaction or the yield produced (entries 2, 5, 7, and 10). O-Succinimide ester 4 failed to react altogether, and while
thioester 5 did produce
the desired product, yields were lower than for the corresponding acid chloride
(3). O-Pfp ester 6 proved to be a suitable acylating agent,
giving 79% yield of the b-diketone within 12 h
and 92% within 24 h. Even better yields and shorter reaction times resulted
from the use of N-acylbenzotriazole
7. Thus, both O-Pfp esters and N-acylbenzotriazoles were investigated in our
subsequent studies. 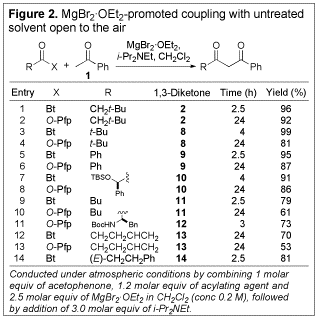 Having
secured a mild and straightforward method for the synthesis of b-diketone 2, we determined the scope of the method with respect to other N-acylbenzotriazoles
and O-Pfp esters (see Figure 2). In general, the N-acylbenzotriazoles outperformed the O-Pfp esters in terms of both reaction time and
yield. The isolated yields were
typically excellent when N-acylbenzotriazoles
were used. Significantly, the
coupling reaction could be carried out in the presence of an acidic urethane
nitrogen protecting group (entry 11), and also in the presence of an enone (entry
14), without detrimental results, as would be expected in the corresponding hard
enolization-based processes.
Having
secured a mild and straightforward method for the synthesis of b-diketone 2, we determined the scope of the method with respect to other N-acylbenzotriazoles
and O-Pfp esters (see Figure 2). In general, the N-acylbenzotriazoles outperformed the O-Pfp esters in terms of both reaction time and
yield. The isolated yields were
typically excellent when N-acylbenzotriazoles
were used. Significantly, the
coupling reaction could be carried out in the presence of an acidic urethane
nitrogen protecting group (entry 11), and also in the presence of an enone (entry
14), without detrimental results, as would be expected in the corresponding hard
enolization-based processes.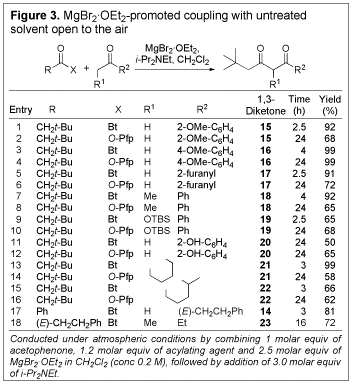
 We
explored the scope of the coupling reaction using a variety of ketones with
various N-acylbenzo- triazoles and O-Pfp esters (see Figure 3). Once again, in all cases the desired 1,3-diketone was
obtained in good to excellent yield.
Notably, the coupling could be conducted with cyclohexanone as the
nucleophile to give the corresponding mono-substituted 1,3- diketone (21)
in excellent yield (entry 13).
Entries 11 and 12 reveal that the process is even compatible with the
presence of phenolic functionality.
Such a substrate would not be amenable to traditional coupling methods
without prior incorporation of a phenol protecting group. A significant result is shown in entry
18 where 1-[(E)-cinnamoyl]-1H-benzotriazole and 3-pentanone could be coupled to
give the desired 1,3-dicarbonyl (23) without subsequent
cyclization to the corresponding 1,3-cyclohexanedione, as is typical of such
systems.
We
explored the scope of the coupling reaction using a variety of ketones with
various N-acylbenzo- triazoles and O-Pfp esters (see Figure 3). Once again, in all cases the desired 1,3-diketone was
obtained in good to excellent yield.
Notably, the coupling could be conducted with cyclohexanone as the
nucleophile to give the corresponding mono-substituted 1,3- diketone (21)
in excellent yield (entry 13).
Entries 11 and 12 reveal that the process is even compatible with the
presence of phenolic functionality.
Such a substrate would not be amenable to traditional coupling methods
without prior incorporation of a phenol protecting group. A significant result is shown in entry
18 where 1-[(E)-cinnamoyl]-1H-benzotriazole and 3-pentanone could be coupled to
give the desired 1,3-dicarbonyl (23) without subsequent
cyclization to the corresponding 1,3-cyclohexanedione, as is typical of such
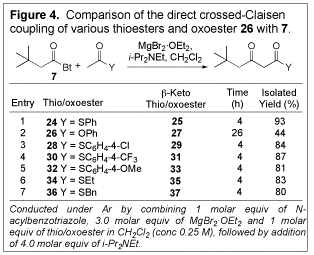
systems.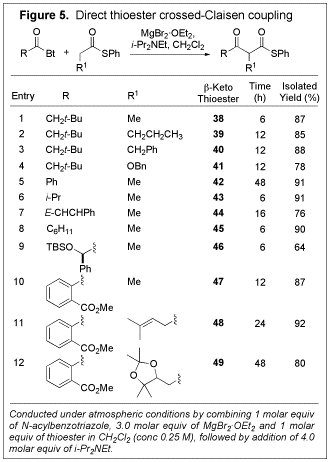 leading
to a controlled direct crossed-Claisen coupling. To test this idea, N-acylbenzotriazole 7 and thioester 24 were
combined in CH2Cl2 in the presence of MgBr2ŠOEt2
and Hunig's base (Figure 4), and the desired product was obtained in excellent
yield (93%). Neither the self-addition products nor the other crossed-Claisen
products were detected. To confirm the importance of the thioesters in this transformation,
oxoester 26 was treated
under analogous conditions. In this case, only a relatively low yield (44%) of b-keto oxoester (27) formed after an extended period of time, thus
confirming the superiority of thioesters in the transformation. Different
thioesters were evaluated for their effect on this coupling reaction; S-phenyl
thioester (24) was found
to be the best. The transformation could be conducted using untreated, reagent
grade open to the air.
leading
to a controlled direct crossed-Claisen coupling. To test this idea, N-acylbenzotriazole 7 and thioester 24 were
combined in CH2Cl2 in the presence of MgBr2ŠOEt2
and Hunig's base (Figure 4), and the desired product was obtained in excellent
yield (93%). Neither the self-addition products nor the other crossed-Claisen
products were detected. To confirm the importance of the thioesters in this transformation,
oxoester 26 was treated
under analogous conditions. In this case, only a relatively low yield (44%) of b-keto oxoester (27) formed after an extended period of time, thus
confirming the superiority of thioesters in the transformation. Different
thioesters were evaluated for their effect on this coupling reaction; S-phenyl
thioester (24) was found
to be the best. The transformation could be conducted using untreated, reagent
grade open to the air. 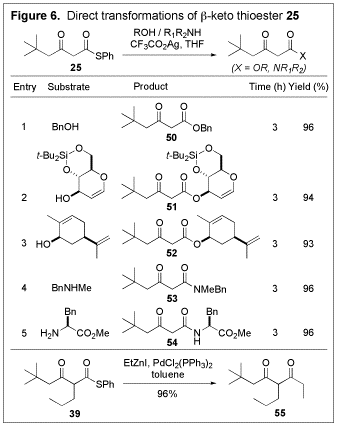 Having
established proof of concept in the direct thioester crossed-Claisen coupling
and confirming that it could be conducted without the need for highly controlled
conditions, we investigated its scope (Figure 5). In general, the
transformation proceeded very well with a range of thioesters and N-acylbenzotriazoles. The reaction conditions
proved to be compatible with a variety of functionality, including ester,
acetal, and a,b-unsaturated
carbonyl compounds. Notably, it even progressed quite well in the case of a
very sterically hindered a-silyloxy substituted N-acylbenzotriazole (entry 9).
Having
established proof of concept in the direct thioester crossed-Claisen coupling
and confirming that it could be conducted without the need for highly controlled
conditions, we investigated its scope (Figure 5). In general, the
transformation proceeded very well with a range of thioesters and N-acylbenzotriazoles. The reaction conditions
proved to be compatible with a variety of functionality, including ester,
acetal, and a,b-unsaturated
carbonyl compounds. Notably, it even progressed quite well in the case of a
very sterically hindered a-silyloxy substituted N-acylbenzotriazole (entry 9).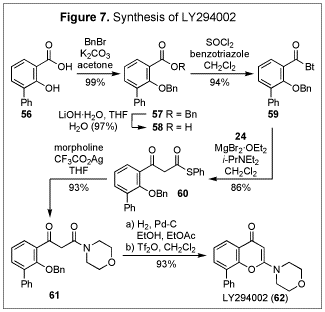 Due to the
unusual reactivity of thioesters, the b-keto
thioesters produced serve as stable synthetic equivalents of b-keto acids and can be converted directly
into a variety of useful compounds under mild conditions (Figure 6).
Due to the
unusual reactivity of thioesters, the b-keto
thioesters produced serve as stable synthetic equivalents of b-keto acids and can be converted directly
into a variety of useful compounds under mild conditions (Figure 6).