42382-B1
Sulfone-Based Methodology for the Construction of Biologically Relevant Furans
Progress has been made in establishing the generality of a novel furan synthesis using the sulfone reagent 2,3-dibromo-1-phenylsulfonyl-1-propene (DBP) in the presence of 1,3-diketones (e.g., 1) and methanolic sodium methoxide.
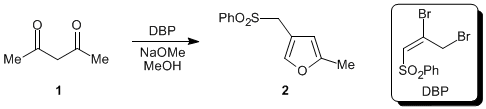
Specifically, one goal was to optimize the newly elaborated phenylsulfonylmethyl furans (e.g., 2) to the corresponding furoic acid derivatives. Toward this end, benzyl phenyl sulfone (3) was used as a model system. In the course of these studies, a moderate counterion effect was observed, with potassium hexamethyldisilazide giving the best results.
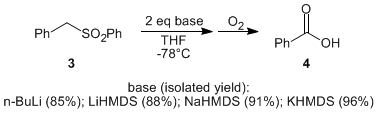
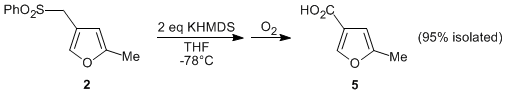
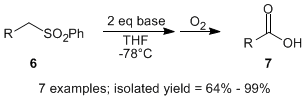
When the optimized conditions were applied to the sulfonyl furan 2, the corresponding 5-methylfuran-3-carboxylic acid (5) was isolated in 95% yield. Encouraged by these results, we surveyed a variety of alkyl phenyl sulfones, and found that the aerobic oxidative desulfonylation was tolerant to a wide variety of substrates, providing carboxylic acids (e.g., 7) in good to excellent isolated yields. Since the starting substrates can be accessed by the alkylation of phenyl methyl sulfone, this represents a new protocol for the synthesis of carboxylic acids from alkyl halides.
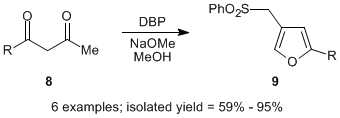
The second area of significant progress in generalizing the sulfonyl-based furan synthesis was with respect to the alkyl group in the 2-position. Here we built upon the observation that when unsymmetrical 1,3-diketones were employed, and a methyl group was attached to one terminus, the acetyl moiety was preferentially cleaved along the mechanistic pathway, leaving the remaining substituent to occupy the 2-position of the furan. This differentiation was remarkably efficient: in the most demanding example (8, R = Me), the selectivity was 5:1; however, in all other cases, the selectivity was > 11:1 and in some instances there was exclusive loss of the acetyl group.
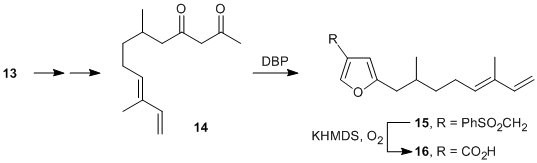
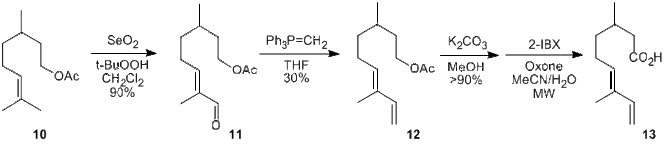
With all the major architectural flexibility established, a study was undertaken to showcase the methodology through the synthesis of the naturally occurring furan 16, which exhibits anti-fouling properties. Three separate synthetic approaches were examined for gaining access to the requisite side-chain for the 2-position. The most successful approach launches from the commercially available citronellyl acetate (10), which undergoes selective allylic hydroxylation and subsequent oxidation in the presence of selenium dioxide and t-butyl hydroperoxide to give enal 11 in excellent yield. Wittig methylenation provides the dienyl acetate 12, which is hydrolyzed under mild conditions and oxidized to the corresponding carboxylic acid (13) with the agency of hypervalent iodine.
The synthetic pathway from carboxylic acid 13 to the synthetic target is straightforward, involving conversion to the acyl chloride, reaction with the anion of ethyl acetoacetate, and subsequent Krapcho decarboxylation to give diketone 14. Our model studies suggest that further conversion to furan 15 and oxidative desulfonylation should proceed smoothly. Current efforts are directed toward this end.