46202-G1
Catalytic Asymmetric Ketone Homologation. Development of Chiral Aluminum Complexes for the Formal Deoxygenative Insertion of Carbonyl Compounds into Acyclic and Cyclic Ketones
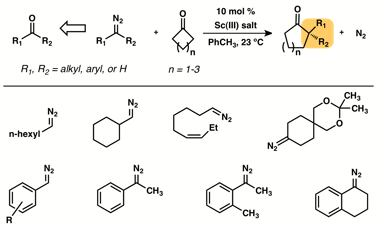
The proposed research for this grant focused on utilizing Al-based Lewis acids to effect a catalytic homologation of non-activated cycloalkanones with substituted diazomethanes. With encouraging preliminary results showing that Al-based Lewis acids cleanly and efficiently promoted the insertion reaction, we examined a number of potential catalysts. Various electron-rich Al- and B-based Lewis acids were studied, but attempts to achieve turnover were unsuccessful. An even greater survey of potential H-bond donors was carried out (alcohols, biphenols, diols, ureas, thioureas, and electronically-activated versions thereof), again with discouraging results. Finally, a screen of lanthanide triflates was highly rewarding. Utilizing the funding from the PRF we demonstrated that scandium (III) salts are uniquely suited to effect catalytic homologation of non-activated cycloalkanones with a variety of aryl- and alkyl-substituted diazomethanes. The results of these studies have been reported in a publication: Moebius, D. C.; Kingsbury, J. S. J. Am. Chem. Soc. 2009, 131, 878-879.
We first examined a series of aryl-substituted diazomethanes and found that the reaction was tolerant of electronic modifications: p-nitrophenyl- and p-methoxyphenyldiazomethane both gave good yields when reacted with cyclobutanone in the presence of 10 mol % Sc(OTf)3. 1-(o-Tolyl)-1-diazoethane also reacted efficiently with cyclobutanone and catalytic Sc(OTf)3 underscoring the remarkable facility with which sterically congested, all-carbon quaternary centers can be installed in one step.
Having demonstrated the utility of this reaction with aryl-substituted diazo nucleophiles, our attention turned to expanding the scope to less stable alkyl-substituted diazomethanes. Initial studies using Sc(OTf)3 as catalyst proved disappointing with much of the mass balance being attributed to Lewis-acid promoted decomposition of the diazo nucleophile. We were gratified to discover, however, that the less Lewis-acidic and more sterically encumbered Sc(acac)3 afforded products and minimized decomposition of the nucleophile. Further studies revealed that Sc(TMHD)3 proved an even superior catalyst for the alkyl-substituted series.
Though the starter grant has been consumed at this point, we were also able to apply its funds towards a study involving aldehyde electrophiles. Specifically, our laboratory has realized a general synthesis of aryl-alkyl and alkyl-alkyl ketones by Sc-catalyzed diazoalkyl insertion into the formyl C–H bond. Mechanistic studies of this transformation confirm that the event proceeds via intramolecular C–H migration in a Sc-complexed diazonium betaine intermediate, thereby setting the stage for development of a catalytic enantioselective variant. These "phase two" results have just been submitted for publication: Wommack, A. J.; Moebius, D. C.; Travis, A. L.; Kingsbury, J. S., manuscript submitted to J. Am. Chem. Soc.
Our methods add both complexity and stereochemistry to carbonyl compounds in one step. Further studies are underway to apply catalytic carbon insertion to the total synthesis of complex molecules in a stereocontrolled manner.