

46485-G7
Origin of Mechanochromism in Polydiacetylene Compound
Research Summary
Polydiacetylene is a unique conjugated polymer. It is known to exist in two separate phases distinguished by drastic changes to the material's optical properties. In one phase, commonly referred to as its “blue phase”, the polymer has a deep blue color and is not fluorescent in the visible range. The other is its “red phase”, which is marked most notably by its bright red/pink color and red fluorescence. Because of both the drastic difference in these colors and the fact that only one phase is fluorescent, this polymer is most desirable as a mechanism for sensor materials and devices.
While the particular uniqueness of polydiacetylene has driven its recent popularity, there remains insufficient understanding about the actual mechanism by which it changes between phases. Early research has given us the exact spatial requirements for photopolymerization to occur between diacetylene monomers and the bevy of recent research leads to some broad hypotheses, but there is still uncertainty in the actual mechanism behind this change. It is believed that the two phases exist as separate conformations to the bond structure of the conjugated polydiacetylene backbone.
For the first year of the project, we have investigated various diacetylene monomers. The surfactant-like diacetylenes were spread out at the air-water interface and subsequently photopolymerized. To systematically investigate the surface pressure at which the color change is observed, the pressure-are isotherm of the Langmuir monolayer has to be completely reversible upon cycles of compression and expansion of barriers. However, the isotherms from these molecules did not show the required reversible feature at all. Two examples are as follows.
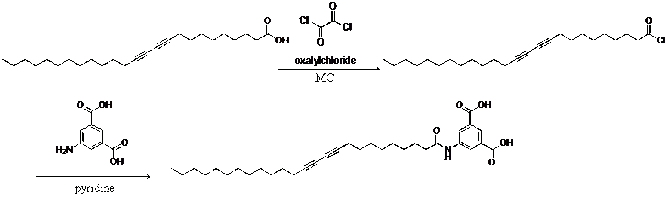
After the reaction between 5-aminoisophthalic acid in 15 mL of pyridine and 10,12-pentacosadiynoic acid chloride, the solvent was removed in vacuo, and the residue was recrystalized in methanol and further purified by silica gel column chromatography (5:1 chloroform: metha- nol) to give 1.00 g as a white solid.
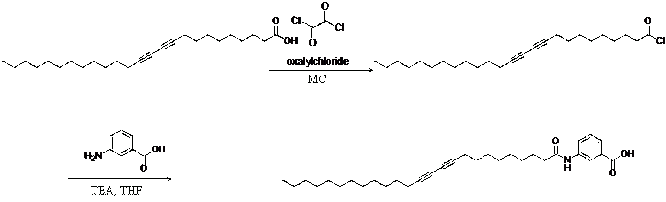
After the reaction between 5-3-aminobenzoic acid in 15 mL of THF and 10,12-pentacosadiynoic acid chloride, the solvent was removed in vacuo, and the residue was precipitated in water and further purified by silica gel column chromatography (5:1 chloroform: metha- nol) to give 0.50 g as a white solid.
We designed a new surfactant-like diacetylene monomer and conducted step-by-step synthesis. The over all scheme is as follows.
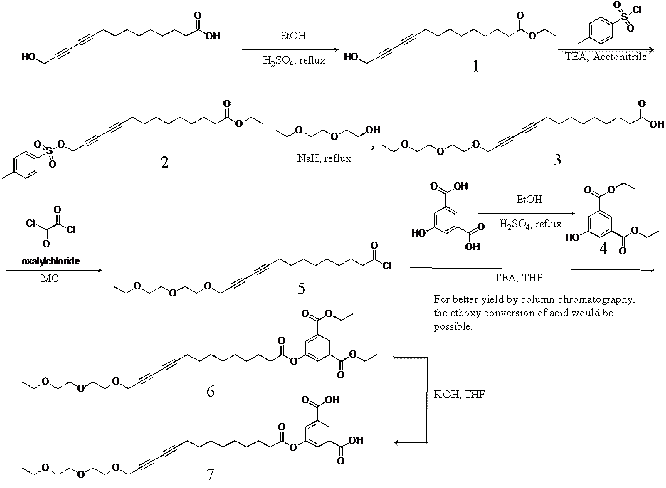
The reaction procedure and the NMR analysis results are as follows.
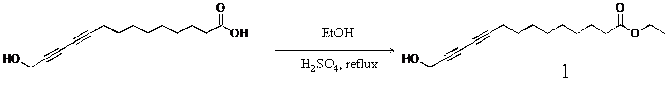
Ethyl 14-hydroxytetradeca-10,12-diynoate(1): To a solution containing 1.00 g (4.23 mmol) of 14-hydroxytetradeca-10,12-diynoic acid in 100 mL of ethnoal was added 0.5 ml (10.57 mmol) of Sulfuric acid at room temperature. The resulting solution was refluxed for 3 h. The solvent was removed in vacuo, and the residue was purified by extraction with diethyl ether and further purified by column chromatography (ethyl acetate : hexane = 1 : 3 v/v) to give 1.28 g (86.2 %) of the desired diacetylene monomer as a pale yellow liquid.
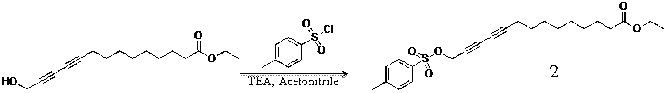
Ethyl tosyl-tetradeca-10,12-diynoate(2): To a solution containing 1.00 g (3.78 mmol) of ethyl 14-hydroxytetradeca-10,12-diynoate(1) in 11.6 mL of acetonitrile was added 0.67 ml (4.87 mmol) of triethylamine at room temperature. To a mixture solution was added dropwise 0.92 g (4.87 mmol) of P-tolunesulfonyl chloride in 5.8 ml of acetonitrile. To residue was charged with nitrogen for 3 min. The resulting solution was stirred for overnight. The solvent was removed in vacuo, and the residue was purified by extraction with diethyl ether.

Diethyl 5-hydroxyisophthalate (4): To a solution containing 3.00 g (16.47 mmol) of 5-hydroxyisophthalic acid in 300 mL of ethnoal was added 4.38 ml (82.35 mmol) of Sulfuric acid at room temperature. The resulting solution was refluxed at 90°C for 3 h. The solvent was removed in vacuo, and the reside purified by extraction with diethyl ether and further purified by column chromatography (ethyl acetate : hexane = 1 : 2 v/v) to give 1.8 g (86.2 %) of the desired monomer as a white solid.
We will finish the synthesis of compound 7 and believe that the new diacetylene will have show a reversible pressure-area isotherm. A fiber optics for in situ monitoring of the color change was purchased. Using the Langmuir method and the designed amphiphilic properties of the compound 7 we will create a monolayer of 7 at the air-water interface and induce their proper assembly by applying the correct surface pressure. We will then expose the layer to 254 nm UV light creating polydiacetylene polymer in its blue phase. By using the fiber optics and applying various surface pressure we will investigate the phase transition behavior and critically important surface pressure in the second year of the project.
Personnel Summary
One graduate student and a postdoc have been partially supported through the PRF-G fund.