45899-G1
Brønsted Acid-Catalyzed Asymmetric Additions to Imines
1. Catalytic Asymmetric Alcohol Additions: A Potential Application to Synthesis.
We have just recently found that During our continued evaluation of non-carbon based nucleophilic additions to imines we have found that alcohols can now be employed, providing the highly enantioselective preparation of chiral N,O-aminals (or N,O-acetals) in good yields. It was quite interesting and unique that in this reaction we could only find improvement in enantioselectivity when we used the pyridinium 9-anthryl-substituted BINOL phosphate derived catalyst PA2 (Figure 1). The product is configurationally stable and isolable. One reason we are excited about this development is due to the scores of natural products and drug candidates (including anti-cancer targets like Psymberin, Pedrin, Irciniastatin A, and (+)-Zampanoide) that have chiral N,O-aminal moiety as a pharmacologically active region (Figure 2). It is widely known that modification of the aminal has drastic effects on the biological activity of these molecules.
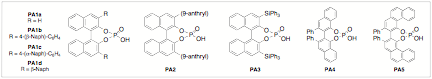
Figure 1. Chiral Phosphoric
Acid Catalysts.
Note: Please refer back to Figure 1 throughout the document for catalyst structures.
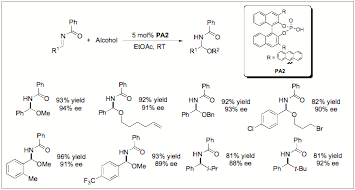
Scheme 1: Preliminary Catalytic Asymmetric
Alcohol Additions to Imines.
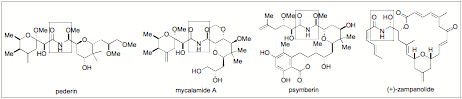
Figure 2: Select Natural Product with Chiral
Hemiaminal Core Structures.
2. The Catalytic Asymmetric Addition of Indoles and pyrroles to Imines.
We have recently begun a program where we have found that nitrogen-heterocycles can be added, in a 1,2 fashion to imines in high yields and with high enantiomeric excess using chiral Brznsted acids as catalysts for the process. During this exploratory study we have identified several heterocycles that are able to perform the imine addition chemistry.
Catalytic Enantioselective Additions. We have now observed excellent enantiomeric excess (ee) when a benzyl group is used to protect the nitrogen of the indole. Our initial investigation has found that PA3 is superior to PA5. This is the first time we have observed a Brznsted acid-catalyzed addition where PA5 is not a superior catalyst. When we first began our study there had not been a reported enantioselective organocatalytic example, to the best of our knowledge, of this chemistry. As we were close to completing our initial screening process for the generality of this reaction two papers were published in this area. The second paper by S. You prompted us to recently submit our results. Both studies required highly activated N-tosyl imines while our procedure uses the more desirable N-acyl imines. Our results, summarized in Scheme 2 below, represent the state-of-the-art for phosphoric acid catalyzed additions of this type. It is clear that we have an excellent reaction to explore with subsequent chemistry to be reported in this area in the future.
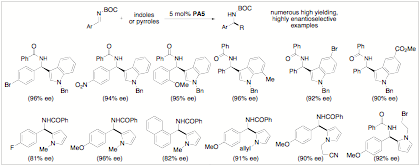
Scheme 2: Representative Examples of a Highly
Enantioselective Brznsted Acid-Catalyzed Addition of Indoles and pyrroles to
Imines.
3.
Brznsted Acid-Catalyzed Enantioselective Reduction of a-Imino Esters.
The catalytic asymmetric reductive amination of ketones is a long-standing, significant problem. Recently it has been shown that such substrates can be reduced with Hantzsch ester using chiral Brznsted acids as catalysts in high ee. We have found that another class of ketimines, those derived from a-ketoesters, can be reduced with excellent yield and ee when using our Brznsted acid catalyst (Scheme 3). We believe that this substrate is an important one synthetically as the product, upon reduction, is an amino acid ester derivative. We have found that the catalyst used previously by MacMillan, for the reductive amination of ketimines, fails completely when used in the reduction of these substrates. Our catalyst (PA5) is superior to those we have compared, and we have shown thus far that good substrate scope can be achieved. For example, we have shown that a variety of a-imino esters with substituted aryl groups can proceed with extremely high ee. We have also shown that primary alkyl substitution on the imine can be tolerated, providing a high yield and ee for the respective a-amino ester.
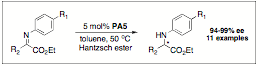
Scheme 3: Catalytic Asymmetric Reduction of a-Imino Esters.
4. Brznsted Acid-Catalyzed
Ring-Opening of Aziridines.
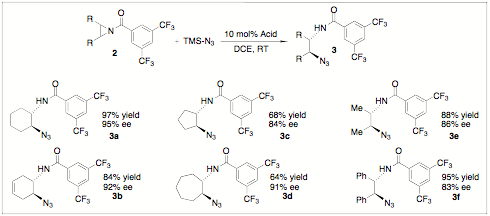
The catalytic asymmetric ring-opening of aziridines is an important, actively pursued area of research. Recently, we have discovered that chiral Brznsted acids can catalyze the ring opening of meso-aziridines with azide to provide a 1,2-diamine precursor in high enantiomeric excess (Scheme 4). These desymmetrization reactions occur at room temperature and with moderate to high yield. Chiral 1,2-diamines have an important value synthetically and our methodology is the first example of a chiral Brznsted acid catalyzing such ring-opening type reactions with high selectivity. Again, PA5 has been shown to be an excellent catalyst in our study. We will continue to evaluate our substrate scope and pursue preliminary studies for the kinetic resolution of racemic aziridines using PA5.
Scheme 4: Brznsted Acid-Catalyzed Ring-Opening Desymmetrization of meso-Aziridines.