

43754-AC7
Electromagnetic Properties of Block Copolymer/Inorganic Oxide Nanocomposite Materials
Electromagnetic Properties of Chemically-Layered Materials
Research Objectives
The goal was to create materials with interesting dielectric properties to tailor absorption of electromagnetic (EM) radiation in frequency-selective fashion. Chemical contrast between dissimilar components can generate charge polarization relaxation at interfaces more intense than relaxations due to molecular dipole re-orientation.[1]
Broadband dielectric spectroscopy (BDS)
BDS can interrogate molecular motions over a wide frequency (f) range.[2] Complex dielectric permittivity e* is given as follows.
e*(f) = e'(f) - ie”(f)
e' and e” are real and imaginary permittivities. e' reflects material polarizability. e” is proportional to molecular energy dissipated per cycle.
A broadband dielectric spectrometer was used over the range 0.1Hz - 3MHz at temperatures (T) from -130 to 200˚ C.
Chemically-layered Materials
Nafion precursor films were modified on one/both sides by reactions of alkyldiamines with SO2F groups. 1200 equivalent weight samples ~100 mm thick were reacted with ethylene diamine (1,2 EDA), 1,2 propylene diamine (1,2 PDA), 1,3 propylene diamine (1,3 PDA) and 1,4 butylene diamine (1,4 BDA). The goal was to create chemically-layered films having SO2F groups on one side and sulfonamide groups on the other side, or sulfonamide groups on both sides with SO2F groups in the middle.
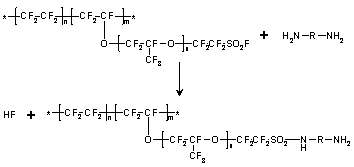 Conversion to the
sulfonamide form proceeds according to the reaction on the left at room
temperature.
Conversion to the
sulfonamide form proceeds according to the reaction on the left at room
temperature.
Due to index of refraction contrast between modified and unmodified regions, optical microscopy can be used to observe reaction depth.
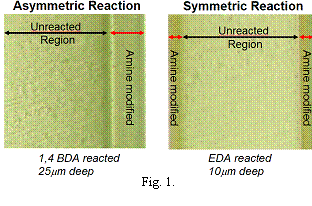 After initial reaction,
films were heated so both amine groups react with SO2F groups forming
cross links.2,3 Optical micrographs of
a film asymmetrically (one side) reacted with 1,4 BDA to a depth of
25mm, and a film
symmetrically (both sides) reacted with EDA to a depth of 10mm both sides are in
Fig.1. These are clearly layered materials.
After initial reaction,
films were heated so both amine groups react with SO2F groups forming
cross links.2,3 Optical micrographs of
a film asymmetrically (one side) reacted with 1,4 BDA to a depth of
25mm, and a film
symmetrically (both sides) reacted with EDA to a depth of 10mm both sides are in
Fig.1. These are clearly layered materials.
FTIR/ATR spectra for unmodified control and 1,2 EDA and 1,4 BDA films symmetrically reacted for 30 min with no curing were obtained. Both sides have the same spectra in each case.
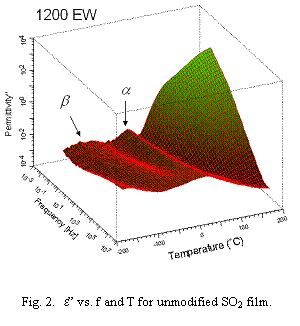
Figure 2 plots e” vs. f and T for an unreacted film. There are five features in order from high to low T assigned to membrane½electrode interfacial polarization, dc onductivity, a (glass transition), b and g relaxations noted earlier.[3] dc conductivity arises from impurity charges. The spectral signature for this conductivity is a linear segment with slope ~1.00 on log10e” - log10 f plots.

|
All three modified films show Tg of the unmodified region and two for amine modified regions.
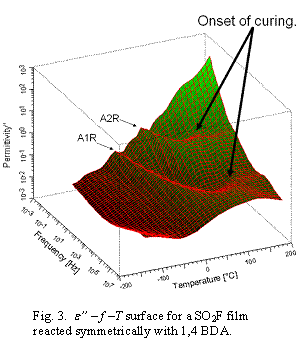
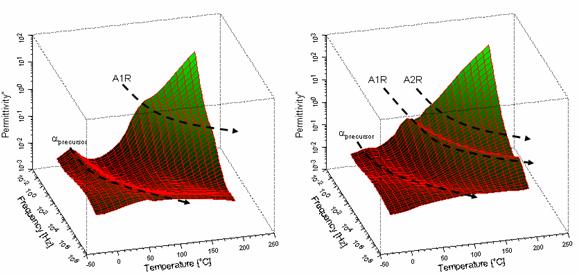
e” – f –T
surfaces for films cured 12h before BDS measurements are in Figure 4: EDA
symmetrically reacted; b) 1,4 BDA symmetrically reacted. Dashed black
curves are crests of relaxation peaks over the T range and are sensitive to
diamine type. A1R relaxation times are lower than those for A2R. Increased
number of relaxations indicates more modes of energy absorption. The sharp
change in e* at the interface has potential to cause
electromagnetic wave reflection as well as inter-layer transmission .
(a) (b)
|

|
|
|
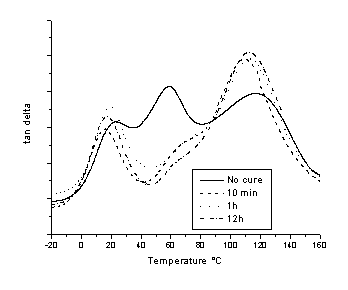
Dynamic mechanical analyses were performed. Generally, for all amines, A1R shifts to DMA spectra shifts to lower temperature. Tan d vs. T at 1 Hz for indicated cure higher T with increasing cure time similar to that of BDS peaks, although A2R in times for 1,3 PDA films symmetrically reacted is in Fig. 5. Tg of the unreacted region shifts to higher temperature, A1R relaxation diminishes in intensity and shifts to higher T and 2R
shifts to lower temperatures while the peak narrows with increased cure time.
|
|
A sulfonyl fluoride perfluoropolymer was reacted symmetrically and un-symmetrically with alkyldiamine molecules to create chemically-layered materials. Optical microscopy confirmed sharp boundaries between distinct layers and FTIR spectroscopy provided evidence of formation of sulfonamide links. BDS indicated appearance of new relaxations attributed to cross-linked layers; these relaxations were also seen in dynamic mechanical analyses.
References