

44401-G1
Investigations into the Reactivity of Vinyl Trichloromethyl Carbinols in the Jocic Reaction
With the invaluable funding made available by the donors to the American Chemical Society Petroleum Research Fund, we have conducted and completed four independent research projects over the past two years. Each of these novel synthetic methods offers a new tool for organic chemists to achieve molecular targets important for materials or medical research in an inexpensive, safer, and more efficient fashion. A synopsis of each is provided.
1. Commercially available Wynberg lactone was used to prepare various asymmetric 2,4-disubstituted butyrolactones in just 3 to 4 steps (Figure 1). Attainment of any possible stereoisomer, based upon commencement from (R)- or (S)-4-trichloromethyl-2-oxetanone, and the capacity to install disparate substituents at C2 make this approach particularly versatile. Such lactones are components of nearly 10% of all biologically active natural products and are also readily converted to valuable asymmetric a,γ-disubstituted carbonyl compounds. The expediency and efficiency of the protocol was highlighted in a synthesis of (+)-harzialactone A in just four steps and 50% overall yield.
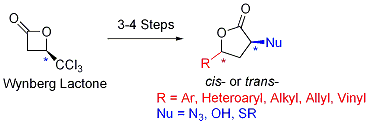
Figure 1. Synthesis of asymmetric 2,4-disubstituted butyrolactones from Wynberg lactone
2. The reaction of aldehydes with trichloromethide followed by sodium borohydride or sodium phenylseleno(triethyl)borate under basic conditions affords homologated carboxylic acids in high yields (Figure 2). This operationally simple procedure provides a practical, efficient alternative to other homologation protocols, and the by-products (sodium chloride and boric acid, after reaction workup) feature a minimal environmental impact. The approach is even compatible with sensitive aldehydes including enals and enolizable α-amino aldehydes, making it an attractive method for carbonyl homologation in complex molecules. It also offers convenient access to α-mono-deuterated carboxylic acids.
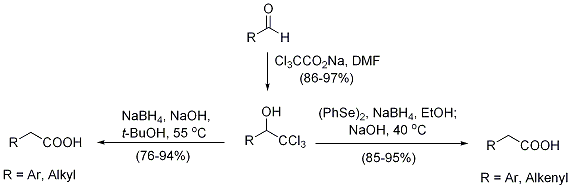
Figure 2. One-carbon homologation of aldehydes to carboxylic acids
3. The tert-butyldimethylsilyloxytrichloromethylmethane (TBSTCM) substituent serves as a readily accessible masking group for aromatic and heteroaromatic aldehydes (Figure 3). The TBSTCM substituent is compatible with a range of common reagents and is hydrolytically stable under both acidic and basics conditions. The functionality offers strategic advantages over other aldehyde protecting groups in that it is orthogonal to acetals, it may be installed concurrently with the silylation of resident hydroxyl substituents, and it may be transformed under mild conditions, along with silyl ethers, in a global desilylation step.
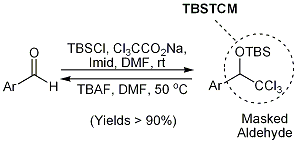
Figure 3. tert-Butyldimethylsilyltrichloromethylmethane (TBSTCM) as a strategically advantageous masked (hetero)aryl aldehyde.
4. Various biologically active natural products and agrochemicals possess homobenzylic alkenes of a defined configuration. Attempts at synthesizing such compounds are often complicated by poor diastereoselectivities during installation of the alkene. We have resolved this issue via a consecutive three-component coupling reaction involving a lithium di[3-(prop-1-enyltrimethylsilyl)]cuprate, variably substituted ortho-arynes, and a selection of common electrophiles (Figure 4). The method affords readily functionalized homobenzylic vinylsilanes with exceptional E-diastereoselectivity and allows for in situ incorporation of carbon- or heteroatom-based electrophiles into the arene. The installed vinylsilanes are conveniently transformed into varied functionalities in a single subsequent operation.
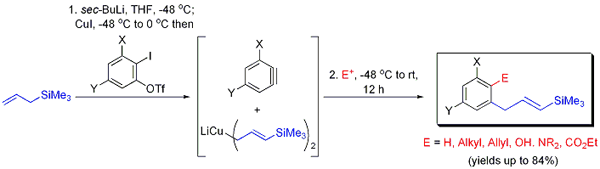
Figure 4. Carbocupration-functionalization of arynes: rapid access to variably ortho-substituted (E)-homobenzylic vinylsilanes.
The gracious contribution from the Donors to the Petroleum
Research Fund has made this research possible.
Without such support, the training and output of the two graduate
students conducting these projects would have been
dramatically minimized, thereby jeopardizing their potential to obtain
desirable post-graduate career or educational opportunities. My scientific output as a tenure-track
assistant professor would have been severely compromised,
and I could not have met University expectations for tenure and promotion. Thus, the