

44989-B1
Stereochemical Studies of Fluorescent Troger's Bases
Scientific Progress:
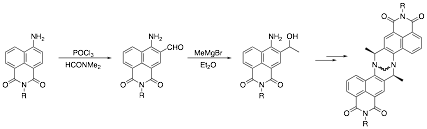
The proposed approach to the synthesis of the monomeric precursors of the required fluorescent Tršger's bases required sequential formylation of an N-alkyl-4-amino-1,8-naphthalimide, Grignard addition to the aldehyde, and optical resolution of the resultant secondary alcohol.
During unsuccessful attempts to use the 3-nitro compound as
a precursor in the photochemical Henry reaction, we observed that the 1H
NMR spectrum of the nitro compound itself showed clear evidence for restricted
rotation about the N—Ca
bond of the 4-butylamino group.
Interestingly, this is not the case when the 3-substituent is bromo (which is physically similar to the bromo group), and we suggest that the nitro compound may
exist as a pair of enantiomeris Impact on the P.I. and students
The grant has provided much needed support to move our work
in the area of fluorescent compounds forward. After the discoveries of new naphthalimide
reactivity last year, the project has become significantly more
problematic. We are following
several new leads for constructing the dibenzodiazocine
ring system. It is important at
this stage to get our observations of heretofore unreported
reactivity in the naphthalimide system into publishable
form, and to publish these results.
We expect this to be a fertile area of research for the future, and to
extend this line of research beyond the end of this grant.
Three students have been directly involved in this project
for some period during the reporting period. Kelsey Dunkle graduated with a
B.S. degree in chemistry, and is pursuing graduate study at North Dakota State
University; her work completing the study of the abnormal bromination
of the N-allyl
systems now has that project ready for publication, and I expect submission of
the manuscript by the end of this year. Elizabeth Raupach continues working
on the project, and is now a senior.
Kyle Kopidlansky has moved onto the project
full-time and is completing the work of Leah Groess
for publication.
Gina Macek, who had a peripheral
association with the project, graduated in May, and is pursuing graduate study
at the University of Connecticut.
 In
the last report, we had not succeeded in incorporating a carbon substituent at
C-3 of the aminonaphthalimide ring system. We have
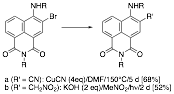
now accomplished this, albeit in lower yields than we would have preferred. In the last report, we noted that the
displacement of bromide from 3-bromo-4,N-dibutyl-1,8-naphthalimide with cuprous cyanide under Ullmann conditions had failed. On further working with the reaction conditions, however, we
have succeeded in displacing the halogen with cyanide: four equivalents of
cuprous cyanide are required, and the reaction requires heating under reflux
for 5 days in DMF. We have also
been successful in incorporating a nitromethyl group
by a photochemical Henry-type reaction.
In
the last report, we had not succeeded in incorporating a carbon substituent at
C-3 of the aminonaphthalimide ring system. We have
now accomplished this, albeit in lower yields than we would have preferred. In the last report, we noted that the
displacement of bromide from 3-bromo-4,N-dibutyl-1,8-naphthalimide with cuprous cyanide under Ullmann conditions had failed. On further working with the reaction conditions, however, we
have succeeded in displacing the halogen with cyanide: four equivalents of
cuprous cyanide are required, and the reaction requires heating under reflux
for 5 days in DMF. We have also
been successful in incorporating a nitromethyl group
by a photochemical Henry-type reaction.
 In
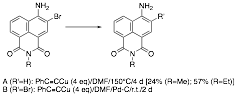
an effort to permit the incorporation of a carbonyl substituent at C-3 without
requiring recourse to redox chemistry that might
affect the naphthalimide ring system itself, we
sought to incorporate an alkynide group at C-3,
expecting that hydration of the triple bond would complete the incorporation of
the C-3 acyl group. Our attempts at incorporating an alkynide
side chain, however, led to reduction of the bromonaphthalimide
when the reaction with cuprous phenylacetylide was
carried out in DMF at elevated temperature, and in recovery of starting
material unchanged when the reaction was attempted in the presence of a
heterogeneous palladium catalyst at room temperature.
In
an effort to permit the incorporation of a carbonyl substituent at C-3 without
requiring recourse to redox chemistry that might
affect the naphthalimide ring system itself, we
sought to incorporate an alkynide group at C-3,
expecting that hydration of the triple bond would complete the incorporation of
the C-3 acyl group. Our attempts at incorporating an alkynide
side chain, however, led to reduction of the bromonaphthalimide
when the reaction with cuprous phenylacetylide was
carried out in DMF at elevated temperature, and in recovery of starting
material unchanged when the reaction was attempted in the presence of a
heterogeneous palladium catalyst at room temperature.
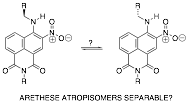 atropisomers.
Our initial attempts to test this were simple chromatography of the
compound on corn starch, and, while the results have
not been reproducible, they have tantalizingly suggested that these atropisomers may, in fact, be resolvable and stable at room
temperature. We are continuing to
see if we can find a better chromatography system (chiral hplc) to resolve this
compound. If successful, this will
represent the first case (to our knowledge) of configurationally stable atropisomers that do not involve a biaryl
moiety at the center of the chiral axis.
atropisomers.
Our initial attempts to test this were simple chromatography of the
compound on corn starch, and, while the results have
not been reproducible, they have tantalizingly suggested that these atropisomers may, in fact, be resolvable and stable at room
temperature. We are continuing to
see if we can find a better chromatography system (chiral hplc) to resolve this
compound. If successful, this will
represent the first case (to our knowledge) of configurationally stable atropisomers that do not involve a biaryl
moiety at the center of the chiral axis.