44949-AC1
Development of Photochemically Removable Groups
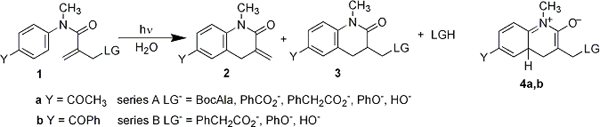
Research investigated the elimination of leaving group anions from zwitterionic intermediates generated photochemically via conrotatory electrocyclic ring closure reactions of methacrylanilides 1a,b. For direct photolysis of 1a in aq acetonitrile containing a buffer at pH 7, the quantum yields for formation of 2a with elimination of the leaving group anions ranged from 0.03 to 0.05 for the series A leaving groups (excluding hydroxide). The photoeliminations were accompanied by formation of lactam 3a with quantum yields of 0.01-0.02. Under the same conditions 1b gave 2b and 3b with corresponding quantum yields of 0.06-0.08 and 0.02 for the series B leaving groups (excluding hydroxide). The leaving groups are likely expelled from zwitterions 4a,b directly, or via the enolate obtained upon deprotonation of 4a,b. Competing with the elimination is 1,5-H shift in 4a,b. The lack of a leaving group effect on the product ratio is better accommodated by the enolate elimination mechanism. Change in solvent to benzene suppresses the photoelimination such that 3a,b becomes the exlusive product, but only when the leaving group is phenoxide. The carboxylate groups are still eliminated in benzene and both 2a,b and 3a,b are observed. The electrocyclic ring closure reaction occurs in the singlet excited state. The triplet excited state of 1b is observed in the 450-750 nm region by laser flash photolysis. The transient is efficiently quenched by 2-naphthalene sulfonate, an efficient triplet quencher. Such quenching has no effect on product quantum yields. The triplet yields are 0.15-0.20 for polar protic solvent and aprotic solvent, as determined by energy transfer experiments using piperylene.