

43048-AC3
Activation of Nitric Oxide at Low-Coordinate Fe, Co, Ni, and Cu Centers
Nitric oxide (NO) is an undesirable product of high-temperature hydrocarbon combustion, reacting swiftly with oxygen in the air to form nitrogen dioxide (NO2), a brown gas which is a principle component of smog, irritating to mucous membranes. With the introduction of automobile catalytic converters, NOx emissions have dropped by 70 – 90 %. These three way catalysts consist of oxide supported late transition metal atoms which both bind NO as well as assist its disproportionation to surface-bound N and O atoms which recombine to give the thermodynamically favored N2 and O2. Nonetheless, the traditional three-way catalysts based on Pt and Rh are not effective in the O2-rich exhaust of diesel and lean-burn engines, motivating new zeolite-based catalysts incorporating less expensive first-row metals.1-3
During the first two years of the award, we have explored nickel b-diketiminate and N-heterocyclic carbene complexes to provide low-coordination environments to host nitrosyl ligands. For instance the three coordinate nickel b-diketiminate [Me3NN]Ni(NO) was structurally characterized and motivated electronic ligand modifications to the b-diketiminate ligand which we explored. In addition, we explored the use of [NHC]Ni(I)(NO) to provide a sterically encumbered coordination environment allowing for the characterization of a number of new three coordinate nickel nitrosyls.
During
the third year of this two-year PRF grant on a no cost extension, we have
further developed the nickel N-heterocyclic carbene chemistry initiated
in the second year of this award as well as begun the exploration of nitric
oxide at higher-coordinate nickel complexes for comparison. In addition, we
have obtained evidence for the formation of a three-coordinate copper-nitrosyl
through detailed studies of b-diketiminato
copper complexes forming the core of the work.
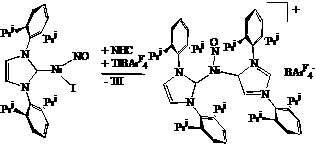
Targeting
the putative two-coordinate {[NHC]Ni(NO)}+ cation we have carried
out the reaction of [NHC]Ni(I)(NO) with TlBArF4 (ArF
= 3,5-(CF3)2C6H3). In the
solution, we observe a new nNO
= 1784 cm-1, modestly at higher energy than the iodide starting
material (nNO = 1766 cm-1).
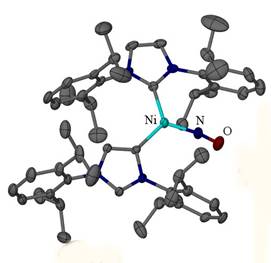
X-ray studies identify the formation of {[NHC]2Ni(NO)}BArF4
in which one of the two NHC ligands is bound through an “backbone” carbon,
representing a “frustrated” NHC-metal bonding mode. Iodide abstraction from [NHC]Ni(I)(NO)
with TlBArF4 in the presence of an extra equivalent of
the NHC ligand provides this cationic nickel nitrosyl in a preparative amount.
The unusual bonding mode of the N-heterocyclic carbene ligand is likely a
result of the steric crowding at the nickel center.
We have also begun to explore differences between NiNO in low-coordinate environments vs. more traditional four coordinate complexes. The tris(pyrazolyl)borate complexe iPr2TpNi(NO) which possesses isopropyl groups in the 3,5-positions of the pyrazolyl rings was also prepared. It will be the subject of future photo-induced linkage isomerism experiments similar to those which have provided evidence for the formation of meta-stable h2-nitrosyls such as [Me3NN]Ni(NO).
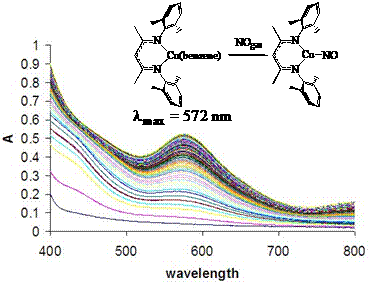
Lastly, we have made observations that suggest the presence of a three coordinate copper nitrosyl [Me2NN]Cu(NO) in solution. Since copper nitrosyl species are implicated in the catalytic disproportionation of NO to N2 and O2 by Cu-ZSM-5,4-6 they represent especially attractive synthetic targets. In particular, the b-diketiminato species [Me2NN]Cu(NO) and [Me2NN]Cu(h2-NO) have been considered by a DFT theoretical study which found that the side-on isomer is 12.5 kcal/mol higher in energy.7
We find that addition of stoichiometric amounts of NOgas to {[Me2NN]Cu}2(benzene) in ether results in the dissolution of the yellow powder to give a deep blue-indigo solution. Following this reaction by UV-vis under dilute conditions results in a growth of a band at 572 nm. While this species has a half-life at room temperature of ca. 1 hour, crystals may be grown at -30 °C which allow for the opportunity to further explore this new species by IR spectroscopy and single crystal X-ray analysis.
 |
References
(1) Witzel, F.; Sill, G. A.; Hall, W. K. J. Catal. 1994, 149, 229-237.
(2) Shelef, M. Chem. Rev. 1995, 95, 209-225.
(3) Millar, G. J.; Canning, A.; Rose, G.; Wood, B.; Trewartha, L.; Mackinnon, I. D. R. J. Catal. 1999, 183, 169-181.
(4) Li, Y.; Hall, W. K. J. Phys. Chem. 1990, 94, 6145-6148.
(5) Anpo, M.; Matsuoka, M.; Hanuo, K.; Mishima, H.; Yamashita, H.; Patterson, H. H. Coord. Chem. Rev. 1998, 171, 175-184.
(6) Datka, J.; Kozyra, P.; Kukulska-Zajac Catal. Today 2004, 90, 109-114.
(7) De Marothy, S. A.; Blomberg, R. A.; Siegbahn, P. E. M. J. Comput. Chem. 2006, 28, 528-539.