

44627-G10
Developing the Aqueous Electrodeposition of Promising Anode Materials for Li-ion Batteries
We have used the second year of PRF funding to pursue a project focused on battery materials. Rechargeable lithium batteries are widely used for portable electronics because lithium is the lightest and most electropositive element critical for high energy density. ADDIN EN.CITE <EndNote><Cite><Author>Tarascon</Author><Year>2001</Year><RecNum>799</RecNum><record><rec-number>799</rec-number><foreign-keys><key app="EN" db-id="2vsvt5eetr2vpne50vrptefpfxfa9vxperwz">799</key></foreign-keys><ref-type name="Journal Article">17</ref-type><contributors><authors><author>Tarascon, J.-M.</author><author>Armand, M.</author></authors></contributors><titles><title>Issues and Challenges Facing Rechargeable Lithium Batteries</title><secondary-title>Nature</secondary-title></titles><periodical><full-title>Nature</full-title></periodical><pages>359</pages><volume>414</volume><keywords><keyword>li battery review</keyword></keywords><dates><year>2001</year><pub-dates><date>15 November</date></pub-dates></dates><work-type>review</work-type><urls></urls></record></Cite></EndNote>1 Pure lithium anodes present a safety hazard due to their explosive reactivity. Intermetallics that can intercalate lithium are an attractive alternative to carbon anodes because they operate several hundred millivolts more positive than the lithium plating potential. ADDIN EN.CITE <EndNote><Cite><Author>Thackeray</Author><Year>2003</Year><RecNum>804</RecNum><record><rec-number>804</rec-number><foreign-keys><key app="EN" db-id="2vsvt5eetr2vpne50vrptefpfxfa9vxperwz">804</key></foreign-keys><ref-type name="Journal Article">17</ref-type><contributors><authors><author>Thackeray, M.M.</author><author>Vaughey, J.T.</author><author>Johnson, C.S.</author><author>Kropf, A.J.</author><author>Benedek, R.</author><author>Fransson, L.M.L.</author><author>Edstrom, K.</author></authors></contributors><titles><title>Structural Considerations of Intermetallic Electrodes for Lithium Batteries</title><secondary-title>J. Power Sources</secondary-title></titles><pages>124-130</pages><volume>113</volume><keywords><keyword>review, lithium intercalation, intermetallics</keyword></keywords><dates><year>2003</year></dates><urls></urls></record></Cite></EndNote>2 Consequently there is no danger of dendritic growth of lithium on the anode. Their main disadvantage is the volume change associated with the conversion of the parent compound to the lithiated counterpart. Repeated cycling pulverizes the particles, resulting in a loss of electrical contact. ADDIN EN.CITE <EndNote><Cite><Author>Tarascon</Author><Year>2001</Year><RecNum>799</RecNum><record><rec-number>799</rec-number><foreign-keys><key app="EN" db-id="2vsvt5eetr2vpne50vrptefpfxfa9vxperwz">799</key></foreign-keys><ref-type name="Journal Article">17</ref-type><contributors><authors><author>Tarascon, J.-M.</author><author>Armand, M.</author></authors></contributors><titles><title>Issues and Challenges Facing Rechargeable Lithium Batteries</title><secondary-title>Nature</secondary-title></titles><periodical><full-title>Nature</full-title></periodical><pages>359</pages><volume>414</volume><keywords><keyword>li battery review</keyword></keywords><dates><year>2001</year><pub-dates><date>15 November</date></pub-dates></dates><work-type>review</work-type><urls></urls></record></Cite><Cite><Author>Thackeray</Author><Year>2003</Year><RecNum>804</RecNum><record><rec-number>804</rec-number><foreign-keys><key app="EN" db-id="2vsvt5eetr2vpne50vrptefpfxfa9vxperwz">804</key></foreign-keys><ref-type name="Journal Article">17</ref-type><contributors><authors><author>Thackeray, M.M.</author><author>Vaughey, J.T.</author><author>Johnson, C.S.</author><author>Kropf, A.J.</author><author>Benedek, R.</author><author>Fransson, L.M.L.</author><author>Edstrom, K.</author></authors></contributors><titles><title>Structural Considerations of Intermetallic Electrodes for Lithium Batteries</title><secondary-title>J. Power Sources</secondary-title></titles><pages>124-130</pages><volume>113</volume><keywords><keyword>review, lithium intercalation, intermetallics</keyword></keywords><dates><year>2003</year></dates><urls></urls></record></Cite></EndNote>1, 2
One way to minimize large volume changes is to use intermetallics that have strong structural relationships to their lithiated counterparts. ADDIN EN.CITE <EndNote><Cite><Author>Thackeray</Author><Year>2003</Year><RecNum>804</RecNum><record><rec-number>804</rec-number><foreign-keys><key app="EN" db-id="2vsvt5eetr2vpne50vrptefpfxfa9vxperwz">804</key></foreign-keys><ref-type name="Journal Article">17</ref-type><contributors><authors><author>Thackeray, M.M.</author><author>Vaughey, J.T.</author><author>Johnson, C.S.</author><author>Kropf, A.J.</author><author>Benedek, R.</author><author>Fransson, L.M.L.</author><author>Edstrom, K.</author></authors></contributors><titles><title>Structural Considerations of Intermetallic Electrodes for Lithium Batteries</title><secondary-title>J. Power Sources</secondary-title></titles><pages>124-130</pages><volume>113</volume><keywords><keyword>review, lithium intercalation, intermetallics</keyword></keywords><dates><year>2003</year></dates><urls></urls></record></Cite></EndNote>2 Cu2Sb reacts with lithium to produce Li3Sb via Li2CuSb. ADDIN EN.CITE <EndNote><Cite><Author>Fransson</Author><Year>2001</Year><RecNum>617</RecNum><record><rec-number>617</rec-number><foreign-keys><key app="EN" db-id="2vsvt5eetr2vpne50vrptefpfxfa9vxperwz">617</key></foreign-keys><ref-type name="Journal Article">17</ref-type><contributors><authors><author>Fransson, L.M.L.</author><author>Vaughey, J.T.</author><author>Benedek, R.</author><author>Edstrom, K.</author><author>Thomas, J.O.</author><author>Thackeray, M.M.</author></authors></contributors><titles><title>Phase Transitions in Lithiated Cu2Sb Anodes for Lithium Batteries: An in situ X-ray Diffraction Study</title><secondary-title>Electrochem. Comm.</secondary-title></titles><pages>317-323</pages><volume>3</volume><keywords><keyword>Cu2Sb, Li intercalation</keyword></keywords><dates><year>2001</year></dates><urls></urls></record></Cite></EndNote>3 The Sb skeleton remains face-centered cubic for all phases, and changes volume by only 94%. Nanoscale dimensions have improved the reversibility of this type of reaction. ADDIN EN.CITE <EndNote><Cite><Author>Li</Author><Year>2001</Year><RecNum>679</RecNum><record><rec-number>679</rec-number><foreign-keys><key app="EN" db-id="2vsvt5eetr2vpne50vrptefpfxfa9vxperwz">679</key></foreign-keys><ref-type name="Journal Article">17</ref-type><contributors><authors><author>Li, N.</author><author>Martin, C.R</author><author>Scrosati, B.</author></authors></contributors><titles><title>Nanomaterial-based Li-ion Battery Electrodes</title><secondary-title>J. Power Sources</secondary-title></titles><pages>240-243</pages><volume>97-98</volume><keywords><keyword>nanowires for Li batteries, SnO2</keyword></keywords><dates><year>2001</year></dates><urls></urls></record></Cite></EndNote>4 We have developed the room temperature electrodeposition of Cu2Sb from aqueous solutions. ADDIN EN.CITE <EndNote><Cite><Author>Mosby</Author><Year>2008</Year><RecNum>520</RecNum><record><rec-number>520</rec-number><foreign-keys><key app="EN" db-id="2vsvt5eetr2vpne50vrptefpfxfa9vxperwz">520</key></foreign-keys><ref-type name="Journal Article">17</ref-type><contributors><authors><author>Mosby, J.</author><author>Prieto, A. L.</author></authors></contributors><titles><title>Direct Electrodeposition of Promising Lithium-Ion Battery Anode Materials: The Case of Cu2Sb</title><secondary-title>J. Am. Chem. Soc.</secondary-title></titles><periodical><full-title>J. Am. Chem. Soc.</full-title></periodical><volume>accepted, 5/9/08, posted on ASAP 7/16/08</volume><edition>7/16/08</edition><dates><year>2008</year></dates><urls></urls></record></Cite></EndNote>5 Electrodeposition is an ideal synthetic method because it can result in crystalline, stoichiometric products with excellent electrical contact to an electrode. We are extending our approach to other anode materials.
CV of 1.0 M aqueous citric acid, 0.1 M Mn(CH3COO)2, and 0.1 M Sb(CH3COO)3. Reference electrode (SSCE), working and counter electrodes (Pt), and scan rate (250 mV/s).
|
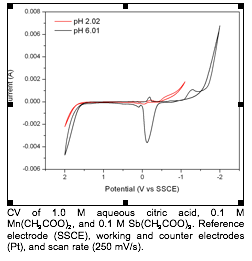 The lithiation of Mn2Sb proceeds
directly to Li3Sb with very little LiMnSb
formation, then cycles almost identically to MnSb.
The cause for this is not understood. Based on the stability of Mn-citrate
complexes in aqueous solution, we attempted the deposition of Mn2Sb.
ADDIN EN.CITE
<EndNote><Cite><Author>Matzapetakis</Author><Year>2000</Year><RecNum>866</RecNum><record><rec-number>866</rec-number><foreign-keys><key
app="EN"
db-id="2vsvt5eetr2vpne50vrptefpfxfa9vxperwz">866</key></foreign-keys><ref-type
name="Journal
Article">17</ref-type><contributors><authors><author>Matzapetakis,
M.</author><author>Karligiano, N.</author><author>Bino,
A.</author><author>Dakanali,
M.</author><author>Raptopoulou,
C.P.</author><author>Tangoulis,
V.</author><author>Terzis,
A.</author><author>Giapintzakis,
J.</author><author>Salifoglou,
A.</author></authors></contributors><titles><title>Manganese
Citrate Chemistry: Syntheses, Spectroscopic Studies, and Structural
Characterizations of Novel Mononuclear, Water-Soluble Manganese Citrate
Complexes</title><secondary-title>Inorg.
Chem.</secondary-title></titles><periodical><full-title>Inorg.
Chem.</full-title></periodical><pages>4044-4051</pages><volume>39</volume><dates><year>2000</year></dates><urls></urls></record></Cite></EndNote>6 As the pH is increased to 6 there is a single distinct anodic peak
present at -1.2 V before the onset of hydrogen evolution. Films deposited at pH
6 and -1.2 V show the codeposition of both metals,
but not the intermetallic compound.
The lithiation of Mn2Sb proceeds
directly to Li3Sb with very little LiMnSb
formation, then cycles almost identically to MnSb.
The cause for this is not understood. Based on the stability of Mn-citrate
complexes in aqueous solution, we attempted the deposition of Mn2Sb.
ADDIN EN.CITE
<EndNote><Cite><Author>Matzapetakis</Author><Year>2000</Year><RecNum>866</RecNum><record><rec-number>866</rec-number><foreign-keys><key
app="EN"
db-id="2vsvt5eetr2vpne50vrptefpfxfa9vxperwz">866</key></foreign-keys><ref-type
name="Journal
Article">17</ref-type><contributors><authors><author>Matzapetakis,
M.</author><author>Karligiano, N.</author><author>Bino,
A.</author><author>Dakanali,
M.</author><author>Raptopoulou,
C.P.</author><author>Tangoulis,
V.</author><author>Terzis,
A.</author><author>Giapintzakis,
J.</author><author>Salifoglou,
A.</author></authors></contributors><titles><title>Manganese
Citrate Chemistry: Syntheses, Spectroscopic Studies, and Structural
Characterizations of Novel Mononuclear, Water-Soluble Manganese Citrate
Complexes</title><secondary-title>Inorg.
Chem.</secondary-title></titles><periodical><full-title>Inorg.
Chem.</full-title></periodical><pages>4044-4051</pages><volume>39</volume><dates><year>2000</year></dates><urls></urls></record></Cite></EndNote>6 As the pH is increased to 6 there is a single distinct anodic peak
present at -1.2 V before the onset of hydrogen evolution. Films deposited at pH
6 and -1.2 V show the codeposition of both metals,
but not the intermetallic compound.We have begun to explore other carboxylic acids, beginning with gluconic acid. The solubility of both manganese and antimony precursors in this acid are high, and the accessible pH range is large. Films deposited at -1.2 V show the presence of Mn and Sb at pH 4, but only Sb at pH 5 (as determined by XRD). CV's of manganese by itself show no electrochemical activity distinct from the gluconic acid, however in the presence of antimony species in solution the codeposition of the two metals is possible. The deposition of the intermetallic is not observed. Future work will determine the speciation present in both solutions as a route toward the direct electrodeposition.
ADDIN EN.REFLIST 1. Tarascon, J.-M.; Armand, M. Nature 2001, 414, 359.
2. Thackeray, M. M. et al. J. Power Sources 2003, 113, 124.
3. Fransson, L. M. L.et al. Electrochem. Comm. 2001, 3, 317.
4. Li, N.et al. J. Power Sources 2001, 97-98, 240.
5. Mosby, J.; Prieto, A. L. J. Am. Chem. Soc. 2008, posted on ASAP 7/16/08.
6. Matzapetakis, M.et al. Inorg. Chem. 2000, 39, 4044.