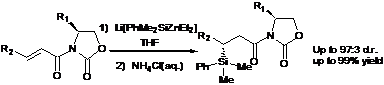41199-AC1
Copper Catalyzed Conjugate Additions of Vinyl- and Silyl Groups: New Tools in Organic Reactions
Presence of catalytic quantities of the copper(I) iodide dimethyl sulfide complex {(CuI)4(SMe2)3} with alkenyl-alkylzincate reagents allows for the complete chemoselective 1,4-addition of various alkenyl groups to a number of a,b-unsaturated carbonyl compounds in CH2Cl2 at +35 °C. The 1,4-addition of the mixed vinylzincate reagent is more efficient than the corresponding vinylzirconocene reagent in CH2Cl2 or THF. By employing CH2Cl2 as a medium, the asymmetric copper-catalyzed addition of the vinyl groups to a,b-unsaturated imides is facilitated by the presence of TMSOTf to give excellent yields and up to 95:5 diastereomeric ratios (d.r.).
The use of Schwartz's reagent {Cp2Zr(H)Cl} in the regioselective hydrozirconation of alkynes is a versatile method for making synthetically useful organometallic reagents. Although organozirconocenes have been used in many applications in organic chemistry, the transmetallation from zirconium to zinc has been demonstrated to be a more efficient subsequent procedure toward the creation of carbon-carbon bonds. In sharp contrast to the more reactive organolithium or Grignard reagents, the corresponding organozinc reagents have distinguished themselves to be more compatible with various functional groups. The process to form such sufficiently nucleophilic alkenylzincate intermediates and their subsequent 1,2-addition reactions to aldehydes was introduced by Wipf, and more recently the enantioselective 1,2-vinylation of ketones was reported by Walsh.
The simple protocol reported herein is initiated by the hydrozirconation of an alkyne using Cp2Zr(H)Cl in CH2Cl2, followed by in-situ transmetallation with Et2Zn. The corresponding alkenylzincate reagent is subsequently exposed to 10 mol% of the CuI·0.75DMS catalyst and the enone. The reaction of the 1-hexenylzincate with benzalacetone utilizing the CuI·0.75DMS complex to give the 1,4-product in 94% illustrates the efficiency of this Cu(I) catalyst. Not only is the formation of the 1,2-addition product completely circumvented in this process, but also the 1,4-addition of the vinylzincate reagent is faster than the corresponding vinylzirconocene reagent in CH2Cl2. Moreover, the copper-catalyzed addition of vinylzincate reagent in CH2Cl2 is not only faster, but the yield of the 1,4-addition product is higher than the corresponding copper-catalyzed 1,4-addition of vinylzirconocene reagents in THF.
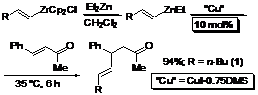
The unique efficiency of the dimethyl sulfide-containing copper(I) iodide complex in the conjugate addition of alkenylzinc reagents is demonstrated using a number of enals and enones. The results exemplify the capability and the synthetic potential of this protocol. It is important to note the efficiency of the Et2Zn reagent in CH2Cl2. Attempts to conduct the reaction using the vinylzirconocene intermediate directly in CH2Cl2, without in-situ transmetallation with the dialkylzinc reagent, resulted in lower yields of the desired products and recovered starting materials.
Because of the increased efficiency of the mixed vinylzincate reagent using CH2Cl2, we broadened the scope of this copper-catalyzed reaction methodology to include a,b-unsaturated imides. We found that the presence of TMSOTf not only safeguards the efficiency of the CuI·0.75DMS as a catalyst in 1,4-addition type reactions of the vinyl group using the mixed alkenyl-alkylzincates to the N-enoyl derived oxazolidinones, but TMSOTf also achieves 1,4-products in high yields as well as diastereomeric ratios up to 95:5. In the absence of TMSOTf, the reaction using the vinylzincate reagents to the imides requires stoichiometric quantities of the CuI·0.75DMS in order to sustain a respectable yield of the 1,4-addition products. The results suggest that TMSOTf is a more efficient Lewis acid with the imides than the corresponding vinylzincate species. In the absence of Et2Zn, the intermediate vinylzirconocene reagent is too unreactive to undergo Cu(I)-catalyzed 1,4-transfer of the vinyl group to the imides in THF, even in the presence of 1 equiv of TMSOTf. Nor is the TMSOTf powerful enough to assist in the 1,4-addition of the vinylzincate reagent in the absence of the CuI·0.75DMS catalyst, which then underscores the crucial role of Cu(I) in these reactions.
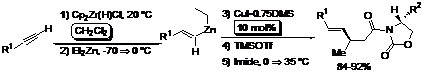
The unique behavior of the CuI·0.75DMS complex and the role of TMSOTf in asymmetric 1,4-additions are currently being explored into further synthetic developments and will be reported in due course.
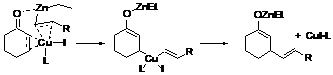
Conjugate addition utilizing various silylzincate reagents [(PhMe2Si)2Zn/LiCl], Li[PhMe2SiZnEt2] and Li[Ph2NEt2SiZnEt2] to a,b-unsaturated carbonyl compounds will be reported accordingly. The [(PhMe2Si)2Zn/LiCl] reagent, which bears similarity as Gilman-type silylcyanocuprate {Li(PhMe2Si)2Cu/LiCN} reagent, guarantees both excellent chemical yield and highly diastereomeric ratio of the 1,4-addition products in the presence of catalytic amount of CuI×0.75DMS. Improvement is also reported by utilizing the monosilylzincate reagent Li[R3SiZnEt2] to conduct conjugate addition reaction which give similar chemical yield and selectivity as disilylzincate reagent, [(PhMe2Si)2Zn/LiCl], reagent even in the absence of the presence of any copper catalysts.