

44844-GB4
Non-Steady-State Kinetic Studies of Bimolecular Nucleophilic Substitution Reactions at Saturated Carbon in Solution
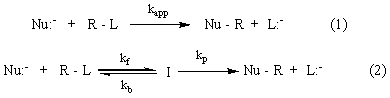
An accepted definition of SN2 mechanism for a reaction in solution is “The nucleophile approaches the substrates from the backside, 180o away from the leaving group. The reaction is a concerted process with no intermediate (eq. 1).” Calculations on the reaction between chloride ion and methyl chloride in DMF suggested that the corresponding ion-dipole reactant complex appears as an energy minimum. 1 Although the ion-dipole intermediate mechanism has also been proposed for some reactions of carbanion nucleophiles in DMSO, 2 whether it is used by common SN2 reactions has not been systematically tested. The aim of the project was to subject a number of SN2 reactions to Non-Steady-State Kinetic (NSSK) studies for the purpose determining whether or not the data are compatible with the classical 1-step mechanism (eq. 1), and if not, to attempt to establish the generality of the 2-step mechanism (eq. 2) for these reactions. The 2-step mechanism accompanies the formation of a kinetically significant intermediate (I) followed by a traditionally-thought concerted SN2 reaction.
The systems that the P.I.'s group, in collaboration with the Parker's group, has subjected to NSSK study during the first year of the two-year project involve the SN2 reactions of the nucleophile p-nitrophenoxide ion in acetonitrile with various substrates including p-nitrobenzyl bromide, methyl p-nitrobenzenesulfonate and methyl p-toluenesulfonate. This year, the analysis of the results collected from last year continued, using the method that have already been employing and the new method (Instantaneous Rate Constant Analysis) that Parker has recently developed 3. The study has also been extended to the reaction of the same nucleophile with m-nitrobenzyl bromide in acetonitrile. We also synthesized another nucleophile, p-nitrothiophenoxide. NSSK studies of the reactions of the latter nucleophile with above substrates in the same solvent are in progress.
The NSSK study of the systems was carried out by means of UV-Vis spectroscopy method together with the contemporary digital processing technology and the computational simulation approach. 3 The kinetic data were collected by following the decay of the nucleophile over a wavelength range from 400nm-550nm. Experimental results collected for all the reactions studied are inconsistent with the simple 1-step mechanism (1). The evidence includes:
i) ii) iii) iv) The comparison of the E.R. - t and kinst –
t data with those simulated for the 2-step mechanism was carried out and good
to excellent fits were obtained. These facts suggest that the SN2
reactions most likely follow the 2-step mechanism. The ion-dipole structure
might be the intermediate for the SN2 reactions, but other
structures cannot be excluded in this stage of the research. Methods such as
UV-Vis and NMR spectroscopy have been applied to search for evidence for the
structure of the intermediates.
Since the project has been granted a one-year
extension, studies of the reactions involving PNPS as nucleophiles are expected
to be completed in the coming year. Resolution of the kinetics of the 2-step
reactions will be carried out and the results will be incorporated into
publications.
Our work suggests that the NSSK method can be
applied to provide mechanistic details for other SN2 reactions. The
results obtained in this research project will greatly enhance the depth of
understanding of relevant organic and biological reactions and eventually
contribute to the pedagogy of fundamental organic chemistry.
Undergraduate students involved in this project
have learned kinetic procedures, basic organic syntheses, compound purification
methods, mechanistic analysis techniques and the use of modern analytical
instrumentation. Since this project involves computational simulation, students
have been trained in using computer programs as well. We believe that the
mastering of the knowledge and the techniques involved in this project will
make students more competitive in their future careers.