

46121-AC3
Systematic and Theoretical Studies of Anion-pi Interactions for the Development of Supermolecules and New Materials
9/1/2007 – 8/31/2008
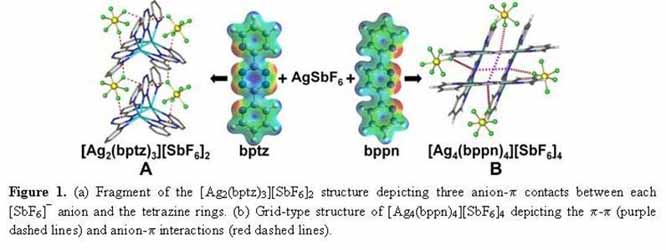
During the past year of this project of the newly recognized anion-p interaction, we have made excellent progress in several of the proposed systems. A comprehensive crystallographic and theoretical study was undertaken to probe the preferred structural motifs of the Ag(I) complexes obtained from the reaction of the Ag(I)X salts (X = [PF6]-, [AsF6]-, [SbF6]-, [BF4]-) with 3,6-bis(2´-pyridyl)-1,2,4,5-tetrazine (bptz) or 3,6-bis(2´-pyridyl)-1,2-pyridazine (bppn), which exhibit different p-acidity of the central rings.[1] The bptz reactions lead to polymeric, dinuclear and propeller-type species (Figure 1a) depending on the anion, whereas the bppn reactions produce the grid-type structures [Ag4(bppn)4]4+ (Figure 1b), regardless of the anion present. In the bppn structures,π-π stacking interactions are maximized, whereas multiple, shorter, and therefore stronger, anion-p interactions between the anions and the tetrazine rings are present in the bptz complexes (shorter by ~0.2 Å than those encountered in the bppn complexes). Furthermore, all the Ag(I) bptz complexes have more than one tetrazine ring p-contact per anion (e.g., in [Ag2(bptz)3][SbF6]2 each anion interacts with 3 tetrazine rings; Figure 1a), whereas the bppn grids have only one pyridazine ring π-contact per anion (Figure 1b). The multiple anion-p interactions per anion established in the case of the bptz complexes (as compared to one per anion in the bppn complexes) and the difference in the preferred structural motifs between the bptz and bppn structures are in accord with the higher p-acidic character of the bptz central tetrazine ring as compared to the more electron-rich bppn pyridazine ring.
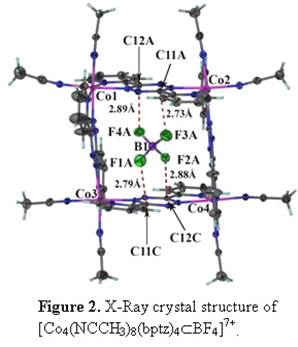
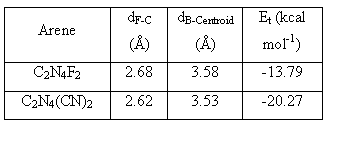
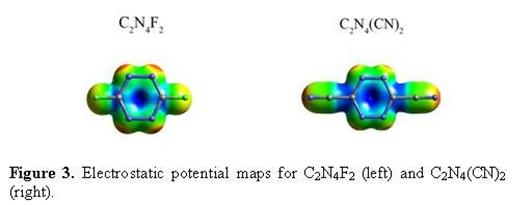
As a continuation of the metallocycles that we have prepared earlier with bptz, we recently isolated the Co(II) analog of [Ni4(NCCH3)8(bptz)4ÌBF4][BF4]7, namely [Co4(NCCH3)8(bptz)4ÌBF4][BF4]7.[2] Single crystal X-ray diffraction studies revealed that [Co4(NCCH3)8(bptz)4ÌBF4][BF4]7 (Figure 2) indeed adopts the square motif with an encapsulated [BF4]- anion in the cage. The anion is positioned in a such a manner that the fluorine atoms of [BF4]- are pointing to the electron deficient carbon atoms of two bptz ligands. The distances between the anion and the tetrazine ring are 2.73-2.89 Å, which are close to the predicted distances from the gas-phase computations for the electron-deficient substituted tetrazine rings C2N4(CN)2 and C2N4F2 (Table 1; Figure 3). Mass spectrometric data revealed the parent ion peak at [Co4(NCCH3)8(bptz)4]8+ at m/z 188.53, an indication that the molecule remains intact in solution.
| Table 1. B3LYP 6-31+G(d’) geometry optimization results for [BF4]-, anion-arene closest contact distances, B to arene centroid distance, and total binding energy (Et). |
Previous
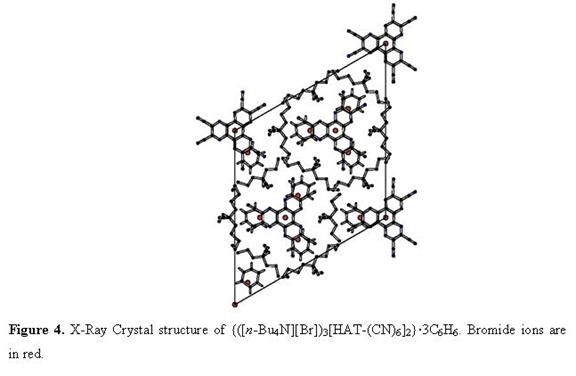
work performed in our group demonstrated that
hexaazatriphenylene-hexacarbonitrile (HAT-(CN)6) is capable of
cocrystallizing in the presence of ([n-Bu4][I])
or cobaltocenium hexafluorophosphate ([CoCp2][PF6]) to
form the intensely colored compounds
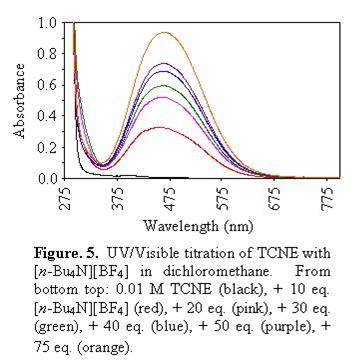
In the same vein, UV/visible spectroscopic studies of the electron deficient molecule TCNE in the presence of [BF4]- ions indicate the appearance of new absorption bands in the visible region (Figure 5).[4] Similar studies are under investigation for 7,7,8,8-tetracyanoquinodimethane (TCNQ), 7,7,8,8-tetracyano-1,2,4,5-tetrafluoro-quinodimethane (TCNQF4) and octacyanoquinodimethane (TCNQ(CN)4). Companion computational studies have also been performed (Figure 6).
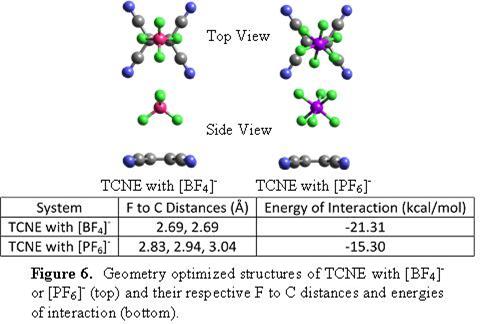
[1]. (a) B. L. Schottel, J. Bacsa, K. R. Dunbar, Chem. Comm., 2005, 46. (b) B. L. Schottel, H. T. Chifotides, M. Shatruk, A. Chouai, J. Bacsa, L. M. Pérez, K. R. Dunbar, J. Am. Chem. Soc., 2006, 128, 5895.
[2]. Giles, I., Dunbar, K. R. et al., manuscript in preparation.
[3] Chifotides,
H.T., [4] Funck,