

45924-GB4
Synthesis and Physicochemical Investigation of Hydrofluorocarbons with Extended pi-Stacked Arene Units
This project aims at the synthesis and physicochemical investigation of hydrofluorocarbons with extended pi-stacked arenes (6) and the evaluation of these molecular scaffolds as hosts for electron-rich arenes or anions.
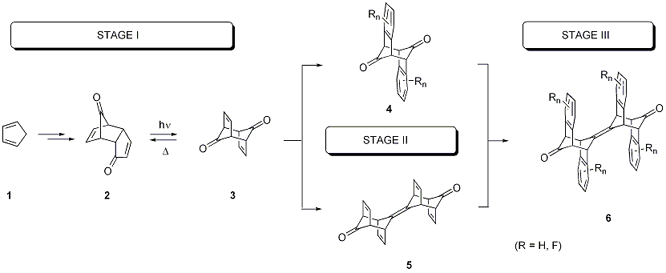
During the first
year we attempted the synthesis of known dienedione 3 [1], the crucial precursor for intermediates of type 4 or 5 and for target frameworks 6,
on a multi-gram scale (STAGE I). Having explored a broad variety of conditions
we only very recently succeeded in optimizing the photochemical conversion of
the endo-cyclopentadienone dimer 2 to its isomer 3 on a preparative scale (Rayonet reactor, 350 nm lamps), thus
having been limited so far to only small quantities of dienedione 3 for any subsequent chemistry.
STAGE II poses several challenges for the selective
functionalization of either the double bonds or the carbonyl units: The chemoselective discrimination between the
two reactivity centers, a one- versus twofold functionalization as well as the
regio- and stereoselective transformation of the target functional group is not
trivial. The thermal lability of
tricycle 3 (back-isomerization to 2) [1] furthermore limits
applicable reaction conditions for a direct conversion of dienedione 3.
Therefore we focused on the functionalization of the previously
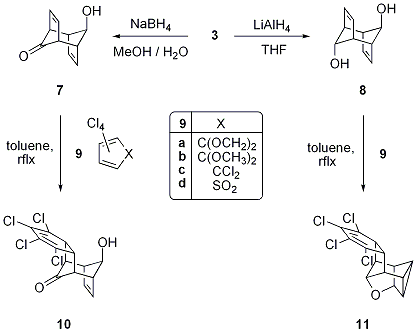
reported, reduced derivatives, monoalcohol 7
and diol 8 [2] of which we submitted
an X-ray structure report.
Besides some rather trivial
conversions of 3 under very mild
conditions (e.g., the double bond epoxidation or methylene addition to the
carbonyl bond) we studied the Diels-Alder reactivity of 7 and 8 with
electron-deficient dienes 9 as a
potential avenue to benzannulated species of type 4. When the
cyclopentadienone derivatives 9a, 9b or hexachlorocyclopentadiene 9c were employed as the diene component
we obtained complex We are currently applying more
forcing conditions for the Diels-Alder reaction of monoalcohol 7 and preliminary experiments indicate
the formation of a not yet fully characterized bis-adduct. Furthermore, we are working on the protection
of both hydroxy and carbonyl groups in 7
and 8, respectively, to allow
possibly cleaner conversion in cycloaddition reactions and to obtain products
that are amenable to subsequent aromatization and dehalogenation reactions.
Once we have
gained complete control of the Diels-Alder reactivity of 7, 8 and/or their
protected derivatives we will employ more valuable, fluorinated building blocks
for the benzannulation and approach STAGE
III toward fluoroarene scaffolds of type 6.
[1] a) U. Klinsmann, J. Gauthier, K.
Schaffner, M. Pasternak, B. Fuchs, Helv.
Chim. Acta 1972, 55, 2643-2659; b) [2] a)
W. Ammann, F. J. Jäggi, C. Ganter, Helv.
Chim. Acta 1980, 63, 2019-2041; b) W. Ammann, C. Ganter, Helv. Chim. Acta 1977, 60, 1924-1925.
 product
mixtures and could not isolate any pure Diels-Alder adducts. In the case of TCTD (9d) though the crude product was rather uniform and after recrystallization
we isolated the mono adducts 10 and 11 from monoalcohol 7 and diol 8, respectively. For both
starting materials the reaction yields a cyclohexadiene derivative after
cheletropic extrusion of SO2 and is accompanied by a transannular
cyclization in case of diol substrate 7. Both new compounds were fully characterized
and their polycyclic frameworks were unequivocally supported by a corresponding
X-ray structure determination.
product
mixtures and could not isolate any pure Diels-Alder adducts. In the case of TCTD (9d) though the crude product was rather uniform and after recrystallization
we isolated the mono adducts 10 and 11 from monoalcohol 7 and diol 8, respectively. For both
starting materials the reaction yields a cyclohexadiene derivative after
cheletropic extrusion of SO2 and is accompanied by a transannular
cyclization in case of diol substrate 7. Both new compounds were fully characterized
and their polycyclic frameworks were unequivocally supported by a corresponding
X-ray structure determination.