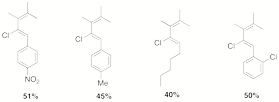45092-GB1
Synthesis and Transition Metal-Catalyzed [3+2] Cycloadditions of Methyleneaziridines
The main focus of the initially proposed work was the synthesis and reactivity of methyleneaziridines. However, as part of our broader interest in the synthetic potential of small highly-strained ring systems we also began investigating the reactivity of cyclopropenes.1 During this work we have uncovered a novel 3,3-sigmatropic rearrangement of the cyclopropenyl acetates 5 (Scheme 1). These cyclopropenes undergo Lewis-acid mediated rearrangement to methylenecyclopropane 2. This transformation represents a new synthesis of methylenecyclopropanes, which have proven to be important and versatile synthetic intermediates.[1] It should be noted that methylenecyclopropanes 6 would be difficult to prepare via other methods.
Divergent reactivity of cyclopropenes 5 has been uncovered upon treatment
with the more powerfully Lewis-acidic TiCl4. When 5 is treated with TiCl4 at
low temperatures chlorodienes 7 are obtained. We propose that this transformation proceeds via loss of the acetate group,
ring-opening results to an allenyl-cation and finally interception of this
cation by chloride from the Lewis-acid. The reaction appears to be selective
for the Z-isomer
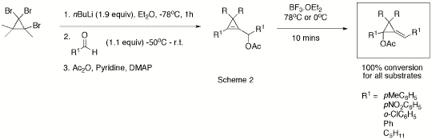
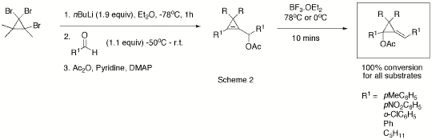
and the stereochemistry of diene 7 was unequivocally shown by X-ray crystallography. A range of cyclopropenes with a pendant acetate group have
been prepared using a modification of Baird's method followed by acetylation
(Scheme 2).[2] This method is ideal as it can be
carried out in one-pot and R, R1 and R2 are easily
varied. Yields steps 1 and 2 varied from 40-85% while the acetylation typically
gave yields from 80-90%. The 1,1,2-trihalogenocyclopropanes required for this
sequence were easily prepared under bi-phasic reaction conditions from
commercially available bromo-alkenes 4.
The
resulting substrates have been used to test the generality of the
3,3-sigmatropic rearrangement and the chloro-diene formation. Interestingly,
the stereoselectivity of the 3,3-sigmatropic rearrangement was found to be
dependent upon Lewis-acid loading and reaction time. Short reaction times and a
full equivalent of BF3.OEt2 resulted in selective
formation of the E-methylenecyclopropane.
In contrast sub-stoichiometric amounts of the Lewis-acid and longer reaction
times led to an equilibrium mixture of E/Z-methylenecyclopropanes. We believe these
observations are a result of the reversibility of the rearrangement, however,
further studies are necessary to fully elucidate the mechanism. The TiCl4-mediated
rearrangement has proved general for a number of substrates (figure 2) and we
are continuing to optimize the yields.
In summary, we have uncovered two Lewis-acid mediated
rearrangements of cyclopropenyl acetates. These transformations contribute
fundamentally to the understanding of how cyclopropenyl acetates behave with
different Lewis acids. In addition, the rearrangements allow preparation of
synthetically important heteroatom-substituted methylenecyclopropanes and
chloro-dienes. While making a
significant impact on the area of cyclopropene chemistry, the two
rearrangements leave a number of questions unanswered and have opened up a
number of avenues for further investigation. In future studies we will
investigate the exact nature of the Lewis-acid interaction to see if it is
activating the oxygen of the acetate or the p-bond of the cyclopropene. We also
plan to develop extend the 3,3-sigmatropic rearrangement to cyclopropenyl
trichloroacetimidates to allow preparation of aminated methylenecyclopropenes.
We believe the TiCl4-mediated rearrangement is potentially powerful
method for preparation of highly substituted dienes, so future studies here
will focus on introducing nucleophiles besides chloride. The research described in this report has had a
significant impact on my career by allowing me to obtain significant initial
results, while opening up two new avenues of investigation. In addition,
the results obtained under the auspices of this grant have already enabled me
to make both oral and poster presentations at the National ACS meeting in
Philadelphia and the Organic Reactions and Processes Gordon Conference in Rhode
Island. Following optimization of the yields for some of the reactions shown
above I plan to submit peer-reviewed publication in the very near future, thus
firmly establishing me in the synthetic community as an independent researcher. Three undergraduate students have been directly involved
with this research project through paid summer research, with one student being
a PRF SUMR scholar. This focused research experience allowed the students to
get a taste of what real research is like and as a result the PRF SUMR scholar
is now planning to pursue a Ph.D. in chemistry. Another two undergraduate
students have worked on the project during the semester and were so excited by
the chance to carry out organic research that they successfully applied for the
NSF REU and NIH MARC (Minority Access to Research Careers) programs respectively.
One of these students is preparing his applications for Ph.D. programs in
organic chemistry and the other will be doing the same in two years. Their decisions to enter graduate
school in chemistry were directly influenced by their involvement with this
project.