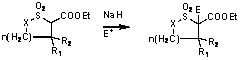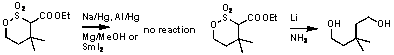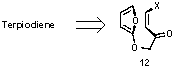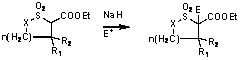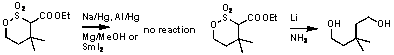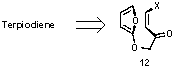Back to Table of Contents
46278-G1
Development of C-H Insertion on Sulfonyl Compounds
Alexei V. Novikov, University of North Dakota
In
the first year of the funding period 3 projects were developed. They were
essential for staring PI's independent career and engaging graduate and
undergraduate students in research. 3 graduate students and 7 undergraduate students
have participated in these projects. PI is currently participating in “Pilot-to-Lab”,
and NATURE programs to also involve high-school and Native American students. 5
presentations on international conferences were made, 2 articles are currently
in preparation for publication, with 2 more planned within the next half a year
that will acknowledge the support of the ACS PRF grant. The
projects performed included development of C-H insertion on sulfonyl compounds
as a synthetic method, synthesis of Plakortethers, and an approach to the
synthesis of Terpiodiene. We
previously discovered new selectivity in intramolecular carbene C-H insertion
on sulfonyl substrates. We have performed a more detailed study of the reaction,
as well as of synthetic transformations of its products. Use
of different rhodium catalysts and variation of the key elements of the
structure were explored. The results are summarized in Table 1. Table 1

| R1,R2,R3,R4 | catalyst | Conditions | Product 2 | Product 3 |
1 | Me,Me,H, H | Rh2(OAc)4 | CH2Cl2,RT | 55% | - |
2 | Me,H,H,H | Rh2(OAc)4 | CH2Cl2,RT | 60%,dr 5:1 | ~3%1 |
3 | Me,H,H,H | Rh2(esp)2 | CH2Cl2,RT | 70%,dr 2:1 | ~3%1 |
4 | Me,H,H,H | Rh2(cap)4 | (CH2Cl)2,reflux | 65%,dr 6:1 | ~3%1 |
5 | Me,H,H,H | Rh2(pfb)4 | CH2Cl2,RT;DBU | 25%,dr 1:1 | 52%1 |
6 | H,H,H,H | Rh2(OAc)4 | CH2Cl2,RT;DBU | ~2%1 | 28%1 |
7 | H,H,H,H | Rh2(pfb)4 | CH2Cl2,RT;DBU | Not detected | 60%1 |
8 | Me,H,Me,H | Rh2(OAc)4 | CH2Cl2,RT;DBU | 50%,dr>10:12 | 20%1 |
9 | Me,H,Me,H | Rh2(OAc)4 | CH2Cl2,RT;DBU | ~5%1 | 60%1,dr>10:12 |
10 | Me,H,Me,Me | Rh2(OAc)4 | CH2Cl2,RT;DBU | ~3%1 | 80%1 |
11 | Me,H,H,H | Rh2(MEPY)4 | (CH2Cl)2,reflux | 55%,ee 2%3 | - |
12 | Me,H,H,H | Rh2(DOSP)4 | CH2Cl2, RT | 50%,ee 10%3 | - |
13 | Me,H,H,H | Rh2(DOSP)4 | Dimethylbutane,RT | 45%,ee 20%3 | - |
14 | Me,H,H,H | Rh2(PTTL)4 | CH2Cl2,RT | 55%,ee 50%3 | - |
1
Combined
yield for both diastereomers 2 Diastereomeric ratio at the b-carbon 3 For the
trans-diastereomer Several
catalysts proved effective for the reaction. Unexpectedly, rhodium
perfluorobutyrate catalyst was found to revert the selectivity back to formation
of five membered rings. Steric
crowding at sulfonyl group (line 10) lead to reversal of the selectivity back
to five membered ring formations, as did deactivation of the insertion site
(lines 6,7). Noteworthy is the good diastereoselectivity at the b-carbon
(the C-H insertion site) observed on substrate secondary sulfonyl substrate
(lines 8,9). Chiral
variation of the reaction was, disappointingly, only moderately successful (The
best ee, obtained with PTTL catalyst, was 50%). Alkylation of the cyclization products
proceeded smoothly, including sulfonate compounds. Notably, complete
diastereoselectivity was observed where formation of diastereomers was possible. Table 2
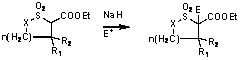
R1,R2,n,X | E+ | Yield |
Me,Me,2,CH2 | Allyl bromide | 95% |
Me,Me,2,CH2 | EtI | 80% |
Me,H,2,CH2 | Allyl bromide | 90%1 |
Me,H,2,CH2 | BnBr | 95%1 |
Me,Me,2,O | Allyl bromide | 85% |
Et,H,1,CH2 | Allyl bromide | 90%1 |
1 Single diastereomer Reductive
scission of the C-S turned out to be much more difficult than that of the
related a-arylsulfonyl esters. Out of common reagents lithium in
ammonia was dound effective, also causing overreduction. Search for milder
reagents and other possible transformations is currently being performed. Scheme 2
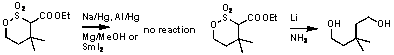
The
results of these studies are currently being prepared for publication. Future
plans include application of the reaction for the synthesis of natural products.
Hepatoprotective antibiotic Bakuchinol is currently targeted. The
second project was the synthesis of Plakortethers – secondary metabolites from
marine sponge Plakortis Simplex (Scheme 3). Scheme 3

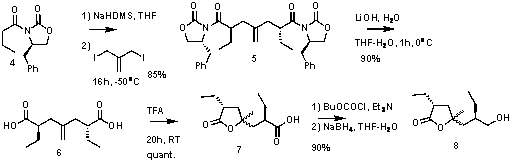
The
synthetic approach reiled on the symmetry of the molecule. C2-symmetric
intermediate, 6, was desymmetrized (Scheme 4). Selectivity could not be
achieved in this transfromation. However, because diastereomers of 8
could be separated and independently utilized, the sequence was still
effectively for preparation of the key intermediate, 10.Scheme 4

After
separation, epimers 10a and 10b were converted to the corresponding
epimeric natural Plakortethers F and G (Scheme 4). The
obtained results are currently being submitted for publication. Synthesis of Plakortethers
A-E is planned in the future, along with biological testing of Plakortethers
and their analogs in collaboration with Fathi Halaweish of South Dakota State
University. Finally,
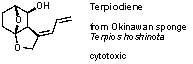
studies on the synthesis of Terpiodiene, an isolate for an Okinawan sponge Okinawan
sponge Terpios hoshinota with cytotoxic activity, were started.
Scheme 5
Initial
plan involved the use of Diels-Alder reaction to construct the tricyclic ketal
core (Scheme 6). However, preparation of the alkoxy furan substrate (12)
proved exceedingly difficult. Scheme 6
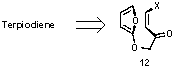
The
new strategy relies on iodocyclization for construction of the cyclic ketal.
Currently, synthetic sequence leading to intermediate 16 has been
developed. Scheme 7

The
future synthetic plan proposes the use of 3+2 nitrile-oxide dipolar
cycloaddition to construct the third ring. In
conclusion, we were able to establish a research program in the area of
synthetic organic chemistry featuring both development of new synthetic methods
and targeted synthesis of natural products. The support by the ACS Petroleum
Research Fund was critical for this endeavor as it was the sole source of financial
support for a period of time.
Back to top