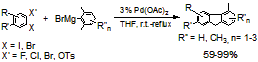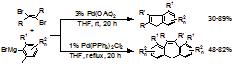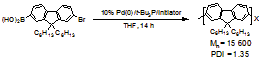44428-AC1
Controlled Pd(0)-Catalyzed Cross-Coupling Polymerizations
In the past funding period, we have continued our study on controlled Pd-catalyzed cross-coupling polymerization processes. We have further studied the “Preferential Oxidative Addition” mechanism for the cross coupling of 1,2-dihalobenzenes/trans-1,2-dibromoethenes with Grignard reagents and further optimize the condition/factors for Pd(0)/t-Bu3P-catalyzed Suzuki cross-coupling polymerization to generate polyfluorenes with controlled length.
We have previously established that dibromobenzenes and diiodobenzene can couple with arylboronic acids via “Preferential Oxidative Addition” mechanism with Pd (0) /t-Bu3P as catalyst. We have also established that the cross-coupling of 1,2-dihalobenzenes with Grignard reagents can occur via “Preferential Oxidative Addition” mechanism when palladium catalysts with phosphine and N-heterocyclic carbene ligands were used. We observed that substituted fluorenes were obtained as the main products when palladium catalysts without phosphine and N-heterocyclic carbene ligands, e. g., Pd(OAc)2, were used, which suggested that benzynes were likely involved as the reaction intermediates. We have thus further studied this fluorene-making process. We found good to excellent yields of fluorenes were obtained for all 1,2-dihalobenzenes and 2-haloaryl tosylates (Scheme 1). Our study provides an efficient method for the preparation of substituted fluorenes, which are potentially useful building blocks in polymer/materials fields, from readily available starting materials.
Scheme 1.
We also
tested the reactions of trans-1,2-dibromoethenes with 2,6-dimethylphenylmagnesium bromides,
in which the benzyne species should be involved as the reaction intermediates. We
found with Pd(OAc)2 as catalyst, substituted
indenes were obtained in good to excellent yields, and with Pd(PPh3)2Cl2
as catalyst, (Z)-tetrasubstituted ethenes were obtained in good yields (Scheme 2). Our study validated the benzyne intermediate
hypothesis for the Pd(OAc)2-catalyzed
reaction of 1,2-dihalobenzenes with hindered Grignard reagents and provides efficient
methods for the preparation of two types of useful organic compounds,
substituted indenes and (Z)-tetrasubstituted ethenes.
Scheme 2.
Based
on our results on “Preferential Oxidative Addition” mechanism and controlled
cross-coupling polymerization from last year, we have further studied Pd(0)/t-Bu3P-catalyzed cross-coupling
polymerization of fluorene-containing monomers. This polymerization was chosen because
polyfluorenes have been demonstrated to be promising light-emitting materials
and 2,7-dibormofluorenes also underwent cross-coupling
with arylboronic acids via “Preferential Oxidative Addition” mechanism. We
initially found with 4-CH3OC6H4Pd(t-Bu3P)Br
as initiator, the lowest polydispersity index (PDI) of the formed polymers was
1.52. During the past funding period, we continued our study and found that
with other initiators, the Pd(0)/t-Bu3P–catalyzed
polymerization could yield polyfluorene polymers with PDI as low as 1.35 (polystyrene
as standard, THF) (Scheme 3). Such
significant PDI improvement suggested that our goal to make conjugated polymers
with the PDI of less than 1.1 by cross-coupling polymerization processes could
be achievable. In addition, our study laid solid foundation for our continuing
study on controlled Pd(0)-catalyzed cross-coupling
polymerization processes.
Scheme 3.