
Back to Table of Contents
47214-AC3
Oxygen Activation by Isopenicillin N Synthase - The Yin and Yang of C-H Activation
Carsten Krebs, Penn State University and J. Martin Bollinger, Penn State University
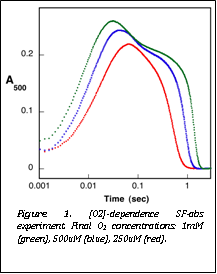
Figure 1. [O2]-dependence SF-abs experiment. Final O2 concentrations: 1mM (green), 500uM (blue), 250uM (red). |
As
previously reported, preliminary results identified an intermediate exhibiting
absorption bands at 320 and 500 nm. Correlation of the stopped-flow data with
freeze-quench Mssbauer kinetic data identified this intermediate as an Fe(IV) species, most likely the Fe(IV)-oxo
intermediate proposed to abstract hydrogen from Cb of the D-Val residue of ACV in the second C-H-cleavage
step in the transformation.
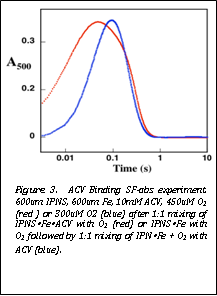
Figure 3. ACV Binding SF-abs experiment. 600um IPNS, 600um Fe, 10mM ACV, 450uM O2 (red ) or 300uM O2 (blue) after 1:1 mixing of IPNSŸFeŸACV with O2 (red) or IPNSŸFe with O2 followed by 1:1 mixing of IPNŸFe + O2 with ACV (blue). |
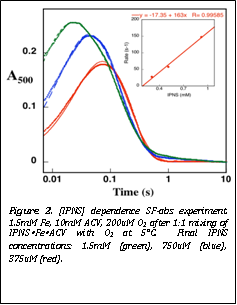
Figure 2. [IPNS] dependence SF-abs experiment. 1.5mM Fe, 10mM ACV, 200uM O2 after 1:1 mixing of IPNSŸFeŸACV with O2 at 5°C. Final IPNS concentrations: 1.5mM (green), 750uM (blue), 375uM (red). |
First,
she showed that the rate of formation of the Fe(IV)
intermediate is linearly dependent on the concentration of O2
(Figure 1). This observation implies that preceding intermediates, including
the proposed superoxo-Fe(III)
complex, do not accumulate to high levels. Rather, the reaction appears
kinetically as a single-step formation of the Fe(IV)
complex followed by its slower decay. From similar experiments under
single-turnover conditions, she could determine that O2 adds to the IPNS„Fe(II)„ACV complex to form the Fe(IV) intermediate
with a second-order rate constant of 160 ± 40 mM-1s-1 and
the Fe(IV)-intermediate then decays with a rate constant of 6 ± 2 s-1
(Figure 2). Stepping backward from these steps, Ms. Tamanaha
probed the kinetics of ACV binding to IPNS„Fe(II) by
mixing this complex simultaneously with ACV and O2 and using the lag
in Fe(IV) intermediate formation as an indication of the time required for ACV
to bind (Figure 3). Simulation of this and similar traces yielded an estimate
of 6 ± 3 mM-1s-1 for association of the enzyme and
substrate to form the O2-reactive complex. Additional replicates
under varying conditions are required to establish that this association is, as
we are currently modeling it, a simple bimolecular association as opposed to a
more complex series of steps involving, for example, an IPNS conformational
change required to attain proper O2 reactivity.
Figure 4. [ACV] dependence SF-abs experiment. 263uM IPNS, 263uM Fe, 1mM O2 after 1:1 mixing of IPNSŸFeŸACV with O2 at 5°C. Final ACV concentrations: 20mM (black), 10mM (green), 5mM (blue), 2.5mM (red). |
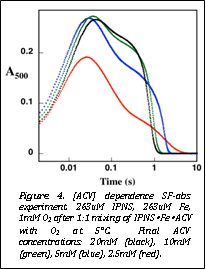
The
fact that productive substrate binding is slow relative to O2
addition allowed Ms. Tamanaha to obtain a good
estimate for the KD of the
IPNS„ACV complex by varying the concentration of the substrate in the
pre-incubation with IPNS„Fe(II) and monitoring the
amplitude of the absorption transient (Figure 4). An initial [ACV] of 5 mM was found to give ~ 67% saturation, allowing a KD of 2 mM
to be estimated. This value is considerably less than the reported KD of ~ 18 mM and, in relation to kinetic parameters discussed above
and below, is much more consistent with reported values of KM for ACV (hundreds of micromolar).
The KD
value can and will be refined to much greater precision by repetition of these
experiments and use of a wider range of ACV and IPNS concentrations.
Figure 5. 4.2-K/zero-field Mssbauer spectra of the IPNS reaction at 5°C. Reaction times are indicated. The Fe(IV) species can be modeled as a quadrupole doublet with d = 0.29 mm/s and ΔEQ = 0.40 mm/s. |
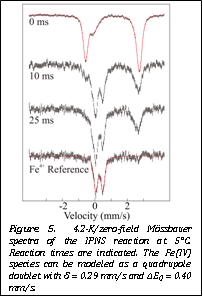
As
mentioned above, we previously reported freeze-quench Mssbauer kinetic data
intended to associate the 320/500 nm-absorbing intermediate with a specific
Mssbauer signature and thereby to diagnose its oxidation state. However, these
data were only preliminary, with only a single time-point along the reaction,
and the intermediate had accumulated only to 17% of the total Fe in the
sample. Since then, Ms. Tamanaha has performed a full time-course and has succeeded
in accumulating close to 60% of the total Fe in the Fe(IV)-intermediate
state (Figure 5).
Currently we are preparing for the second phase of our IPNS
mechanistic dissection. We have
contracted with Bachem, Inc. to synthesize ACV per-deuterated at the Cβ of the L-Cys in an effort to slow decay of the first C-H-cleaving
intermediate, the postulated superoxo-Fe(III)
complex. If the 320/500
nm-absorbing intermediate is, as we have assigned, the Fe(IV)-oxo species, then the A-(b-[2H]2-C)-V
should retard formation of the intermediate but not perturb its decay kinetics.
More importantly, use of the labeled substrate should allow the first
C-H-cleaving complex to accumulate for spectroscopic detection and
characterization.
Back to top






