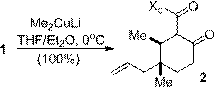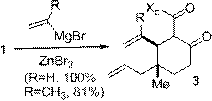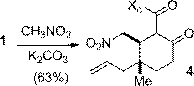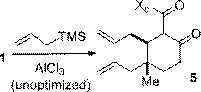43238-AC1
Sequential Birch Reduction-Allylation/Cope Rearrangement for the Enantioselective Construction of Carbocyclic Quaternary Stereogenic Centers
Reactions to extend the utility of chiral 4,4-disubstituted 2-cyclohexenones were explored, including conjugate addition and reductive-alkylation. The chiral 4,4-disubstituted 2-cyclohexenones, such as 1, were readily available from the Birch-Cope sequence (Scheme 1), a process developed in our lab with ACS-PRF support. The combination of conjugate addition and alkylation reactions with the Birch-Cope sequence will afford exciting new avenues to efficiently construct bioactive natural products, which are an important source of inspiration for new medical therapies.
Scheme 1. Birch-Cope sequence.
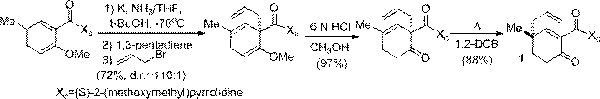
A selection of conjugate additions was developed to extend the utility of 1 towards the synthesis of complex natural products. Efficient and stereoselective reactions were realized with cuprate addition (Scheme 2), zinc-mediated conjugate Grignard additions (Scheme 3), and nitromethane conjugate addition (Scheme 4). A Sakurai reaction with 1 was also successful although the yield has yet to be optimized. All four reactions generated cyclohexanone products containing functionalized vicinal quaternary-tertiary stereocenters which should be useful intermediates in the synthesis of complex bioactive structures.
Scheme 2. Cuprate conjugate additions.
| Scheme 3. Zinc-mediated Grignard conjugate additions.
|
Scheme 4. Nitromethane conjugate addition. | Scheme 5. Sakurai reaction |
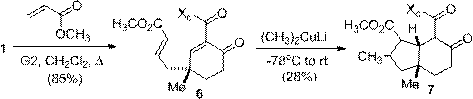
A tandem conjugate-Michael addition was accomplished by first functionalizing the terminal alkene in 1 by olefin cross metathesis (Scheme 6). Addition of methyl cuprate to 6 lead to a tandem conjugate-Michael addition and a modest yield of 7. The hydrindane product 7 is a commonly used core in natural product synthesis. It is almost exclusively generated by Hajos-Parrish-Eder-Sauer-Wiechert reaction, therefore the generation of a complimentary enantioselective approach to hydrindane structures should prove quite valuable.
Scheme 6. Olefin cross metathesis and tandem conjugate-Michael addition.
Finally, the synthesis of cyclohexanone structures with two stereogenic quaternary centers was achieved through a reductive-alkylation of 1 (Scheme 7). Cyclohexanone structures with two quaternary centers form a particularly challenging core in the field of natural product synthesis and the reductive-alkylation of 1 has the potential to help address the shortage of enantioselective methods to these structures. Another long-term goal in the reductive-alkylation studies is to combine this procedure with the conjugate addition studies previously described. Coupling a conjugate addition reaction to 1, with an alkylation of the C-2 of the cyclohexanone, would stereoselectively generate three contiguous chiral centers (i.e. C-2, 3 and 4), including two quaternary centers.
Scheme 7. Reductive-alkylation of 1.
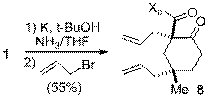
The activities of the past year were undertaken by the principal investigator, William P. Malachowski; a post-doctoral fellow, Sanjeev Kumar; two graduate students, Tina Morgan Ross and Jisun Lee; one post-baccalaureate student, Jonathan Bennett; and three undergraduate research assistants, Sarah Tabi, Sarah Miller and Iva Yonova. The principal investigator has benefited tremendously with a significant increase in research productivity as a result of the resources, both personnel and supplies, obtained through PRF funding.
The post-doctoral fellow, Sanjeev Kumar, worked on the grant for one month and he was able to hone valuable skills in enantioselective and natural product total synthesis. In August 2008, Sanjeev moved to a new industrial post-doctoral position for a different synthetic chemistry experience. It is his long-term goal to find a full-time position in the pharmaceutical industry.
Jisun Lee, a graduate student,
spent one semester conducting research associated with the PRF award. She is
engaged in continuing education studies at the The undergraduate researchers have likewise been exposed to
methodology development in the field of asymmetric synthesis. They have undergone significant growth in
their ability to conduct synthetic chemistry research and they have learned a
range of synthetic techniques from reaction preparation and set-up to product
purification and analysis. One of the
students (Iva Yonova) has graduated and is currently
enrolled as an organic chemistry graduate student at the We continue to pursue the application of the Birch-Cope
sequence and related synthetic tools to the efficient creation of complex
bioactive molecules and we have applied to the National Institutes of Health to
obtain further funding to continue this line of research with a particular focus
in the field of antibiotic development.