45897-GB1
Chromium Carbene-Accelerated Type II Intramolecular Diels-Alder Reactions as a Gateway to Strained Ring Libraries
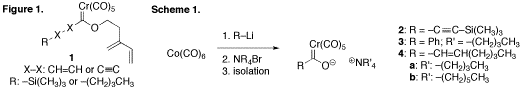
At the time of this report, our research has been focused on the preparation of Fischer carbene-containing type II intramolecular Diels-Alder substrates 1 (Figure 1). Unfortunately, our efforts have met with more difficulty than was expected, given the seemingly sound body of literature that supported our proposed routes. Initially, alkynyl complex 2 was targeted using a variety of reported procedures (Scheme 1). These attempts proved to be unfruitful, as no product was ever isolated. In an attempt to discover the root cause of these problems, we decided to redirect our efforts towards the synthesis of perhaps the best-documented Fisher carbene complex, phenyl derivative 3. Once again, several different procedures and combinations thereof were applied to the synthesis before complex 3 was finally isolated in 53% yield, as its tetrabutyl ammonium salt. With this success in hand, efforts returned to the preparation of complex 2. Utilizing the procedure that had been optimized for complex 3, complex 2 again failed to form.
Discouraged and in the hopes
that success might be achieved with the reportedly more stable alkenyl
complexes 4, we
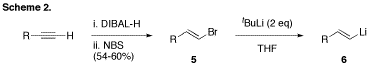
shifted our focus again. Alkenyl complex 4 had not been our original target, as it employs a vinyl
lithium reagent 6 that
itself requires several additional synthetic steps to prepare and necessitates
the use of tert-butyl
lithium – a reagent we had hoped to avoid (Scheme 2). However, utilizing
vinyl lithium 6 under
similar conditions to those applied to the synthesis of complex 3, we were finally able to isolate one of
our targets, alkenyl complex 4a, as its tetrabutyl ammonium salt in 68% yield. Further
investigations sought to improve the yield and stability of this complex by
utilizing other tetra alkyl ammonium bromides (i.e. NMe4Br, NHex4Br,
NEt3BnBr, and NMe3DecylBr). Gratifyingly, by using the
larger tetrahexyl ammonium counterion, salt 4b could be prepared in good purity and 90%
yield. Additional derivatives of complex 4, containing different R groups, also performed well under
these optimized conditions, yielding the desired salts in >80% yield in all
cases.
With
this success in hand, our efforts shifted to coupling an alcohol to our newly
formed carbene salt 4b
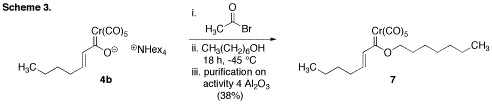
(Scheme 3). Using methods from the literature, we attempted to couple complex 4b
to 1-heptanol; however,
under a variety of different conditions <10% of the desired carbene complex 7 was observed. In an effort to improve
these results, a systematic study of both the experimental and purification
conditions was conducted. Through these efforts, it was discovered that complex
7 could be formed in a
workable 38% yield when acetyl bromide was used as the activator, the reaction
was allowed to stir for an extended period of time at -45 °C, and purification
was accomplished on deactivated basic alumina.
While the yield of the
coupling reaction is still lower than we would like, we were pleased at the
progress that had been achieved and were confident that this approach would
provide access to the desired Diels-Alder substrates 1, when the required diene-containing
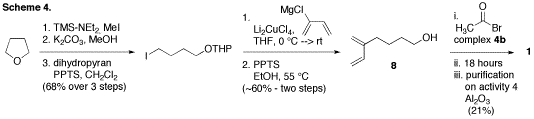
alcohol 8 was
utilized. To this end, a single attempt was made to couple alcohol 8 to complex 4b at the end of the summer under the best
coupling conditions (Scheme 4). We were pleased to observe a 21% yield of the
desired alkenyl derivative 1, thus demonstrating that, while further optimization is required,
our method is a viable approach to the synthesis of this class of Fischer
carbene complexes.
Future work will continue to focus on
optimizing the coupling between alcohol 8 and complex 4b as well as related derivatives. The compounds produced through
these efforts will be used to assess the feasibility of the type II
intramolecular Diels-Alder reaction. If successful, the strained ring library that
results will be the first in a series of compound families designed
specifically for the evaluation of alkene distortion. Although the term of this
grant has ended, work in this area is continuing in our labs and will be
reported with appropriate acknowledgement in due course.