45440-B1
Exploiting the Oxacalixarene Scaffold: Structural Diversity, Macrobicyclic Hosts, Multicalixarenes, and Molecular Tweezers
Research Summary September 2007 – September 2008
The Katz research group continues to investigate
the synthesis and molecular recognition properties of oxacalixarenes
incorporating dichloronaphthyridine 2.
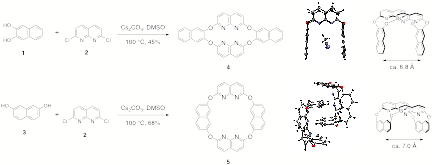
Oxacalix[4]arenes derived from this electrophile, formed in one step by
nucleophilic aromatic substitution reactions, adopt 1,3-alternate solid-state
and solution conformations. This conformation creates a U-shaped cavity with a
spacing between opposing walls of approximately 7ü, a distance that allows a
guest molecule residing in the cavity to simultaneously interact with the
pi-surfaces of both aromatic walls.
Molecules with this topology are known as "molecular tweezers", and
potentially have binding affinity toward aromatic guest molecules that experience
favorable interactions with the interior pi-surface of the cavity. Our initial investigations into the
synthesis and host properties of these molecular tweezers were recently
published in Chemical Communications ( We have begun to develop a
second-generation approach to molecular tweezers derived from
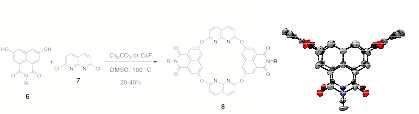
2,7-dihydroxynaphthalimides 6. Incorporation of this nucleophile
allows the formation of molecular hosts with both enhanced solubility and
increased pi-surface relative to systems 4 and 5, above. An X-ray
structure of 8 has given us
confirmation that a 1,3-alternate conformation is maintained using tricylic
nucleophile 6. We are using NMR spectroscopy,
fluorometry, and isothermal titration calorimetry to study the molecular
recognition properties of oxacalixarenes 8. The fluorescence properties
are especially relevant in the development of oxacalixarenes 8 as chemical sensors.