47103-AC4
New Model Systems to Measure Weak Non-covalent Interactions
The goal of the project is to use small molecule model systems to measure weak non-covalent interactions in order to better understand the variables that influence their stability. Non-covalent interactions such as hydrogen bonds and arene-arene interactions are important in determining the folding of proteins, the specificity of biologically active molecules, and in the selectivity of organic transformations. A better understanding of the strengths of these non-covalent interactions would allow us to more accurately predict and design better systems for the above applications.
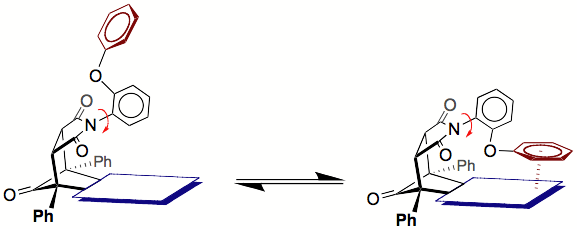
Our approach to measuring very weak non-covalent interactions is to design a molecular balance, which is a molecule that is in dynamic equilibrium between two different conformational states (shown below). In the "folded" conformation, the molecule can form intramolecular non-covalent interactions. In the "non-folded" conformer, the molecule cannot form the non-covalent interactions. Thus, by measuring the folded/unfolded ratio, the strength of the intramolecular non-covalent interaction can be measured with high accuracy.
In
the first year of the grant, we have redesigned our molecular balance system
and have focused on measuring weak arene-arene interactions. Our initial design was based on
N,N'-diarylureas and N,N-diarylamides.
However, these proved difficult to synthesize and they also isomerized
too quickly to evaluate at room temperature. Therefore, an N-arylimide based system (shown below) was
designed, synthesized, and studied to measure the intramolecular arene-arene
interactions between the red phenyl arm and the blue arene-shelf.
This new systems had a number
of attractive characteristics:
1)
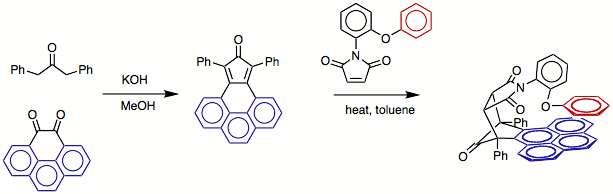
The balance with easily prepared in two steps via a double Aldol cyclization,
followed by a Diels-Alder reaction as shown below.
2) Both the phenyl arm and the arene-shelf
could be easily varied due to the modular nature of the synthesis.
3)
The rotational barrier about the Nimide-Caryl bond was
sufficiently high (~27 kcal/mol) that the folded and unfolded conformers were
under slow exchange at room temperature.
Thus, their ratio was easily measured by integration of the
corresponding peaks in the 1H NMR spectra.
4) Finally, the balance system was soluble
in a wide range of solvents and thus, the influence of solvent effects on the
arene-arene interaction could be measured.
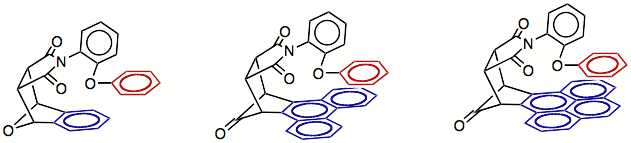
Our initial studies
with this system measured the strength of arene-arene interaction for a series
of balances in which the size of the arene shelf was varied (shown below). As expected, the strength of the
arene-arene interaction increased with the size of the shelf due to greater
overlap of the two surfaces. In
addition, x-ray crystallographic studies confirmed that the arene-arene interaction
in the folded conformer adopts a face-to-face geometry due to the rigidity of
the bicyclic framework. This is in
contrast to other molecular balance systems in which the orientation of the
arene surfaces is not well defined.
The influence of solvents
on the strength of the arene-arene interactions were examined. As expected, the strength of the
interaction increased with increasing polarity of the solvent. However, it was surprising that this
solvent induced folding was of similar magnitude for all three balances systems
that were examined. This suggests
that the strength of the arene-arene interaction in the folded state remains
and the only thing that is changing is the solvation energy of the unfolded
state.