45801-AC4
Bond Compression in Triptycene-Containing In-Cyclophanes
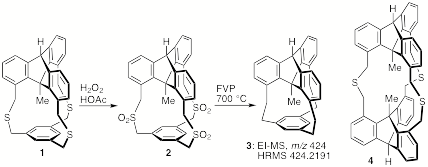
in-Cyclophanes. This award chiefly supports the synthesis of highly strained in-cyclophanes with compressed covalent bonds. Most of the effort thus far has been spent on the synthesis and reactions of in-methyl cyclophanes such as 1 and 2. The C-CH3 bond distance in cyclophane 2, which we reported previously,[1] is about 1.48 ü (X-ray) — about 0.05 ü less than the standard distance for a sp3-sp3 C-C bond. To further compress the bond, one or more sulfur atoms must be extruded, and relatively large amounts of thioether 1 and sulfone 2 have been required for the development and optimization of these reactions. The ultimate target is hydrocarbon 3, where B3LYP/6-31G(d) calculations suggest that the C-CH3 bond distance will shrink to 1.43 ü.
Thus far, all attempts to shorten the arms of compound 1 by means of Stevens rearrangements (both the
conventional Stevens and the benzyne-Stevens reactions) have proven
unsuccessful, with no evidence of any contracted molecules. However, flash vacuum pyrolysis of trisulfone
2 at 700 ░C has given very
promising results, if only in low yield thus far. A hydrocarbon has been isolated from these reactions that
shows a clean, correct EI-MS and HRMS for compound 3, as well as a reasonable 1H NMR spectrum
(complicated by dynamic processes typical of such cyclophanes). The synthesis of additional material
and crystallization attempts are in progress. The synthesis of double in-methyl cyclophane 4 requires similar precursors, but
thus far the macrocyclization reactions have yielded only compounds with two,
rather than the necessary three, linked arms.
Diradical Cyclizations. This award has also supported a study
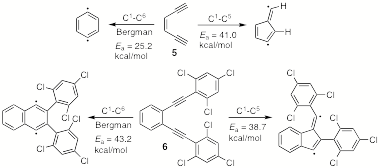
of a novel diradical cyclization of an enediyne. In the course of the synthesis of a polyphenylene cyclophane,
we observed the unexpected formation of a benzofulvene by the apparent C1-C5
cyclization of a diaryl enediyne.
For simple enediynes, the conventional Bergman (C1-C6)
cyclization predominates, as shown by the BLYP/6-31G(d)-calculated Ea's
for the C1-C5 and C1-C6
cyclizations of compound 5.[2] However, our own computational studies
indicated that the inclusion of terminal aryl groups both lowers the C1-C5
transition state by means of the mesomeric stabilization of an incipient
radical and elevates the C1-C6 transition state by means
of steric congestion. In the most
extreme case, bis(2,4,6-trichlorophenylethynyl)benzene (6), BLYP/6-31G(d) calculations indicate that the C1-C5
cyclization is strongly favored over the Bergman reaction. This was verified experimentally by the
synthesis of 6 and its thermal
cyclization to the corresponding benzofulvene.[3]
[1] Song, Q.; Ho, D. M.; Pascal,
R. A., Jr., J. Am. Chem. Soc. 2005, 127, 11246-11247. [2] Prall, M; Wittkopp, A.;
Schreiner, P. R. J. Phys. Chem. A 2001,
105, 9265-9274. [3] Vavilala, C.; Byrne, N.; Kraml, C. M.; Ho, D. M.;
Pascal, R. A., Jr. J. Am. Chem. Soc. 2008,
in press. [ASAP Article, DOI:
10.102/ja803413f]