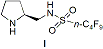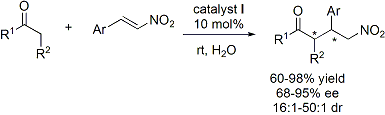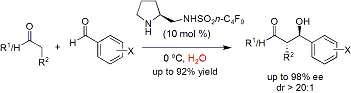Back to Table of Contents
44804-G1
Recyclable and Reusable (S) Pyrrolidine Sulfonamide Organocatalyst for Asymmetric Synthesis
Wei Wang, University of New Mexico
Environmental concerns associated with chemical processes
have encouraged the development more environmentally friendly (greener)
organic reactions. In recent years,
reactions that take place in the environmentally clean, safe and cheap solvent
water have received considerable interest.
A great deal of effort has gone into the development of asymmetric
organic reactions in aqueous media with a main focus on processes promoted by organometallic substances.
Recently, organocatalysis has emerged as a new
field in asymmetric synthesis. With the
scope of this field rapidly expanding, it is important to recognize the
potential limitations and disadvantages associated with the use of organocatalysts.
Typically, organocatalyzed processes are
carried out in organic solvents. The
development of organocatalytic enantioselective
reactions that take place in water remains a challenging task. In addition,
high organocatalyst loadings (10-20 mol%) are generally required in order to
complete the transformations in reasonable timescales. Paralleling this is the high cost of the chiral materials used to prepare the organocatalysts,
which is a major concern especially when the catalysts are used for a large
scale of reactions. Owing to these
limitations, the development of recyclable and reusable organocatalysts
that promote reactions in water is a significant goal needed to be addressed in
order to expand applications of organocatalysis as
part of environmentally benign approach to fine chemical synthesis. To this end, we recently developed a
recyclable and subsequently reusable chiral fluorous pyrrolidine sulfonamide (I) that catalyzes highly enantioselective Michael addition reactions of ketones and aldehydes with nitroolefins in water (Figure 1).
Figure
1. Reusable
Chiral Fluorous Pyrrolidine Sulfonamide Organocatalyst
(I).
Recently, the fluorous chemistry has emerged as a powerful strategy for
facilitating catalyst recovery.
Introducing a fluorous tag into a catalyst can
make it easily recoverable by using simple fluorous
silica gel (silica gel with a fluorocarbon bonded phase)
based solid-liquid extraction. By
design, a n-C4F9
tag was incorporated into organocatalyst I so that it could be easily separated
employing this technology. To test this
feature, I (20 mol% employed in order to ensure the accuracy of evaluating catalyst recovery)
was used to promote the Michael addition reaction of cyclohexanone with trans-b-nitrostyrene. Importantly, I is cleanly recovered (>90%) from
the reaction mixture by using fluorous solid-phase
extraction and the catalyst can be repeatedly reused. In each reuse, the recovered catalyst retains
its high activity, and high levels of enantioselectivity
(90% ee) and diastereoselectivity (≥33:1 dr) even after 6 cycles.
Having established that I
can be easily separated and repeatedly reused, we next probed its use as a
catalyst for a wide range of Michael addition reactions between ketones and aldehdyes and nitroolefins (Figure 2).
The results showed that the reactions proceeded efficiently (60-98% yield) with high to excellent levels of enantioselectivity
(68-95% ee) and diastereoselectivity (≥16:1 dr).
Figure 2. Recyclable and Reusable Organocatalyst I in Promoting Michael Addition of Ketones and Aldehdyes to trans-b-Nitrostyrenes.
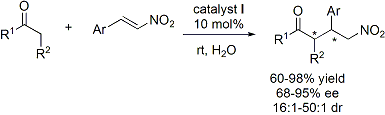
This work has been published on Org. Lett., 2006, 8, 3077-3079. This highly impacted work has received
considerable interest from scientific community. It has been recognized as “Hot Paper” in
March 2007 and the one of most cited articles (top 6)
in 2006 on Org. Lett. It has also been highlighted in http://www.organic-chemistry.org/abstracts/lit1/320.shtm.).
In our continuing effort aimed at expanding the scope of the
organocatalysis, recently, we have successfully
demonstrated that the catalyst fluorous (S) pyrrolidine
sulfonamide I can promote highly enantioselective aldol reactions
of ketones and aldehydes
with aromatic aldehydes in water (Figure 3). Again, the catalyst can be recovered from the
reaction mixtures by simple fluorous solid-phase
extraction and subsequently reused (up to 7 cycles) without a significant loss
of catalytic activity and stereoselectivity. This work has been published on Org. Lett., 2008, 10, 1211-1214.
Figure 3. Recyclable and Reusable Organocatalyst I in Promoting Aldol
Reactions of Ketones and Aldehydes
with Aldehydes.
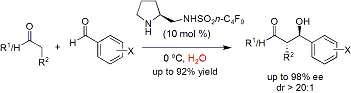
Back to top