
Back to Table of Contents
42087-AC5
Studies of the Interfaces between Conjugated Oligomers and Self-Assembled Monolayers
James E. Whitten, University of Massachusetts (Lowell)
Significance
of this Project
The goals of this project are to understand
the interaction of conjugated oligomers, such as sexithiophene, with other
adsorbed organic layers, including self-assembled thiol monolayers. The
motivation for this work is that overlap of electronic states between organic
layers often dictates the performance of electronic devices, including
light-emitting diodes and solar cells. Alkanethiol monolayers are being used to
"tune" the work function of electrodes for organic electronic devices, and
organic layers are usually deposited on top of these electrodes.
Because of their surface sensitivity, X-ray
and ultraviolet photoelectron spectroscopies (XPS and UPS) are ideal for
studying interfacial chemistry and charge transfer when one layer is step-wise
deposited onto another. This PRF-supported project has benefited the Whitten
research group in that it has funded a Ph.D. level graduate student and enabled
us to perform our first studies of organic-organic interfaces. It is noteworthy
that while interfaces between organic layers and metals have been extensively
investigated, organic-organic interface studies are relatively sparse.Summary
of Progress Because
of its potential importance for photovoltaics and field-effect transistors, we
have performed most of our studies using sexithiophene (6T); its chemical
structure is shown in Figure 1. Experiments in which
this molecule is thermally deposited in ultrahigh vacuum (UHV) onto
Buckminsterfullerene (C60) films and self-assembled thiol monolayers (SAMs) on
gold surfaces have been performed. In the case of a fullerene film, charge
transfer from 6T to C60 is evidenced by shifts in the highest occupied
molecular orbitals (HOMOs) of both molecules, with the HOMOs of C60 and 6T
shifting to lower and higher ionization energies, respectively. 6T
and thiophene monomer have also been deposited on self-assembled,
fluorine-functionalized thiol (1H,1H,2H,2H-perfluorodecanethiol, hereafter
referred to as PFDT) monolayers on gold substrates cooled to 135 K. The
fluorine atoms serve as "tags" in XPS experiments and enable us to deduce the
extent of penetration of the monolayer. In some cases, thiophene has been
adsorbed (instead of sexithiophene) by simply leaking it into the UHV chamber
and condensing it on the cold, organic monolayer-covered surface. The binding energy of
the F 1s XPS peak decreases following thiophene and 6T adsorption,
indicating charge transfer to the fluorinated SAMs. UPS measurements
substantiate charge transfer, with the valence features of thiophene and 6T in
contact with the monolayer appearing at higher ionization energies compared to
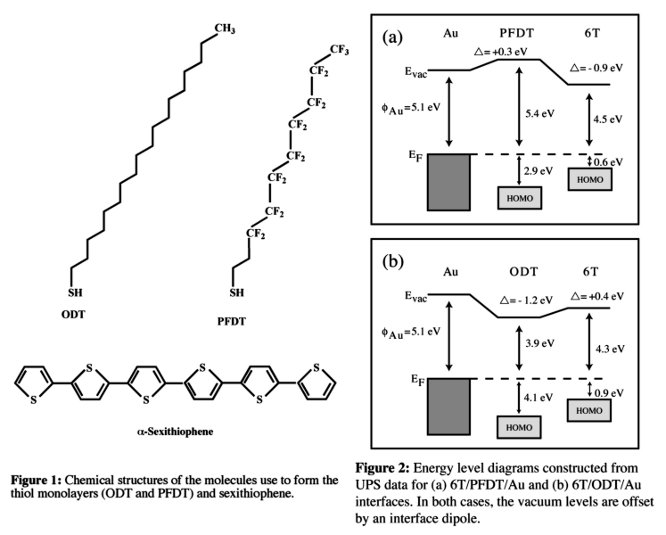
thicker layers. The energies of the UPS-measured vacuum levels of 6T deposited
on PFDT/Au illustrate the absence of a common vacuum level between the organic
layers at the PFDT-6T interface and the presence of a -0.9 eV interface dipole.
Similar measurements performed for 6T deposition on self-assembled
octadecanethiol (ODT) give a weaker interface dipole of opposite sign
(+0.4 eV). The relatively large value and sign of the 6T/PFDT/Au interface
dipole suggest that charge transfer to the PFDT-covered surface results in the
formation of dipoles with their negative ends toward the Au surface, in
contrast to the 6T-ODT interface. These data permit construction of the energy
level diagrams in Figure 2.
 The effects of a SAM layer on X-ray-induced
oligomerization, which is known to occur for condensed thiophene, were also
investigated. Comparison of the thickness of oligomeric thiophene formed by
1253.6 eV X-ray irradiation on clean and PFDT-covered gold surfaces
demonstrates that a thicker oligomer layer forms on the SAM-covered surface,
suggesting that the spacing provided by the SAM reduces quenching of electronic
excitations that lead to X-ray-induced oligomerization.
The effects of a SAM layer on X-ray-induced
oligomerization, which is known to occur for condensed thiophene, were also
investigated. Comparison of the thickness of oligomeric thiophene formed by
1253.6 eV X-ray irradiation on clean and PFDT-covered gold surfaces
demonstrates that a thicker oligomer layer forms on the SAM-covered surface,
suggesting that the spacing provided by the SAM reduces quenching of electronic
excitations that lead to X-ray-induced oligomerization. This
project also involved two other studies related to organic interfaces. A method
of patterning a dibutylphosphonate-substituted, soluble form of 6T with
nanoscale lateral dimensions has been developed. The method consists of forming
a template of a hydrophilic thiol monolayer (e.g., a carboxylic terminated
alkanethiol) by microcontact printing or dip pen nanolithography. The remainder
of the surface is "backfilled" with a hydrophobic thiol. When the soluble,
somewhat hydrophilic form of 6T is spin-coated on the surface, it selectively
adsorbs on the hydrophilic regions. A second "subproject" involves measuring
the effect of the substrate work function on the XPS core levels of an organic
layer. By depositing potassium on top of an alkanethiol monolayer-covered
surface, the work function of the surface can be modified. It is found by XPS
that the C 1s core level of the carbon atoms in the alkanethiol chains are
pinned to the vacuum level instead of the Fermi level. However, the sulfur atoms
that are near the gold surface remain pinned to the Fermi level.


