46387-GB3
Enantioselective Dearomatization Agents Based on Chiral C2-Symmetric Ligands
We are investigating a series of novel d6 molybdenum and tungsten complexes involving chiral C2-symmetric tetraamine ligands. It is our hope that this work will lead to the development of enantioselective dearomatization agents. We note the recent development of pi-basic d6 molybdenum and tungsten dearomatizing metal fragments by Harman et. al. as well as various applications of chiral C2-symmteric tetraamine ligands in enantioselective syntheses. To date, ligands of the type trans-N,N'-bis(heterocycle-2-methyl)cyclohexane-1,2-diamine have been investigated primarily in the context of small, first-row transition metals, and we suspect that heavy-metal complexes of such ligands may have applications beyond those of the current project.
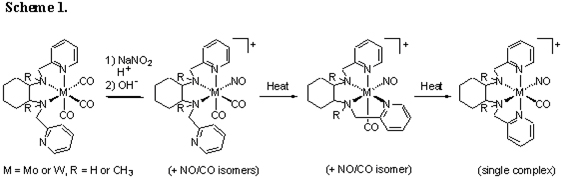
Prior to the current grant cycle, we established the reaction of trans-N,N'-bis(heterocycle-2-methyl)cyclohexane-1,2-diamine ligands with molybdenum and tungsten hexacarbonyl to give tridentate tricarbonyl complexes. For some variations of these tridentate complexes, we were able to show that nitrosylation followed by heating gives corresponding tetradentate mononitrosyl monocarbonyl complexes. Depending on the specific metal and ligand involved, some reactions gave a cis-beta ligand topology, while others gave the desired cis-alpha topology, in which the C2-symmetry of the ligand is retained.
During the past year, we have determined that the above reaction proceeds by substitution of a single linear nitrosyl ligand for a carbonyl ligand (Scheme 1). The corresponding tridentate mononitrosyl dicarbonyl complexes can be observed for molybdenum variations and isolated for tungsten variations. For complexes involving pyridine-based versions of the tetraamine ligand, heating drives these tridentate mononitrosyl dicarbonyl complexes initially to the corresponding tetradentate cis-beta mononitrosyl monocarbonyl complexes and then to the corresponding tetradentate cis-alpha mononitrosyl monocarbonyl complexes. The cause of the thermodynamic preference for the cis-alpha form is not known, but the complex identities are well-supported by 1H NMR and IR data as well as several x-ray structures.
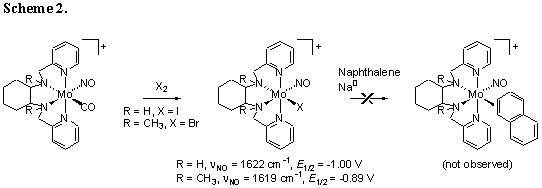
Two versions of the molybdenum tetradentate
cis-alpha mononitrosyl
monocarbonyl complexes have been oxidized using iodine
and bromine to give the corresponding d5 tetradentate
cis-alpha mononitrosyl
monohalide complexes, which we view as potential
direct precursors to dihapto aromatic complexes
(Scheme 2). Unfortunately, attempts to
bind aromatics by reducing these complexes in the presence of naphthalene and
other substrates have not been successful.
IR and electrochemical data suggest that the associated tetradentate mononitrosyl
pi-basic metal fragments are not quite as electron-rich as those which have previously
been demonstrated to form dihapto complexes with
aromatics. Data from our tridentate
complexes and tetradentate mononitrosyl
monocarbonyl complexes suggest that the corresponding
tungsten species are more electron-rich than their molybdenum congeners, and we
hope that this trend will hold true for the corresponding d5
tungsten halides.
ACS-PRF funds spent during the past year were used to
purchase our second MBraun Unilab
purge inert atmosphere glovebox. This box is used primarily for the above research
project and as such has allowed, in a very practical way, for greater involvement
of undergraduate research students in the project. Two students who participated in this project
during the 07-08 academic year had sufficient research
experience to successfully compete for NSF-REU fellowships during the summer of
2008. The box is also used by students
in our senior-level inorganic chemistry teaching lab, and in this way the
ACS-PRF support has benefited about a dozen students and will continue to
enhance the educational experience of many more in years to come.
We expect that a short paper detailing the synthesis and
characterization of several of the above complexes will be submitted for
publication shortly.