45324-GB1
Electrochemical Reduction of Cinnamyl Bromide in the Absence and Presence of Nitric Oxide at Carbon Cathodes in Acetonitrile
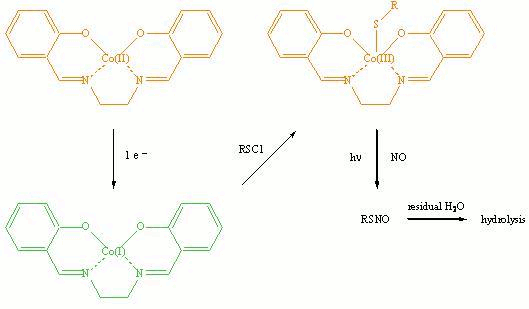
A simple route with mild experimental conditions, as shown below, has been used to synthesize thionitrites (RSNO) by using electrogenerated cobalt(I) salen as the mediator.
However, due to the presence of residual
amount of water in acetonitrile (ACN), which was used
as the solvent for the electrolyses, the thionitrites
quickly underwent hydrolysis1 and decomposed. Consequently, the pure
products were not obtained. Alternative methods are being explored to minimize
the amount of water in the solvent as well as the corresponding reaction with thionitrites.
On the other
hand, the electrochemical behavior of cinnamyl
bromide has been studied. The direct reduction of cinnamyl
bromide at carbon cathodes in ACN gives either carbanions
or radical intermediates, depending upon the selected potential. Table 1 shows
the coulometric data and product distributions for
the reduction of 10 mM cinnamyl
bromide at carbon cathodes in ACN containing 0.050 M tetramethylammonium
tetrafluoroborate (TMABF4) at 1.85 V or
1.35 V (vs. SCE). In the absence of proton donors (diethyl malonate,
DEM), similar n values and product
distributions are observed for both reduction potentials. With the addition of
200 mM DEM, the product distribution changes
significantly when the substrate is reduced at 1.85 V. The product yield of beta-methylstyrene
increases and that of dimer greatly decreases.
Additionally, the diethyl ester of cinnamyl malonate, which is the trap adduct formed by the reaction
between the substrate and DEM, is found in the yield of 36%. In contrast, at
the reduction potential of 1.35 V, the yield of dimer
remains about the same and only a small amount of the trap adduct is generated
when DEM is added. These experimental results are very similar to the electrochemical
reduction of benzyl iodide, indicating that carbanions
are produced at 1.85 V and mostly radicals are formed
at 1.35 V.2 Table 1.
Coulometric data and product distributions for
controlled-potential electrolyses of 10 mM cinnamyl bromide at reticulated vitreous carbon electrodes
in ACN containing 0.050 M TMABF4
Reduction potential / V Added agent n Product distribution / %a 1 2 3 4 5 6 7 Total 1.85b 1.07 3 20 79 trace trace trace 102 200 mM DEM 1.13 4 32 33 36 105 1.35b 1.05 3 22 75 trace trace 100 200 mM DEM 0.97 3 15 73 13 104 a 1 = allylbenzene; 2 = beta-methylstyrene (cis:trans = 2:98); 3 = dimer (alpha-alpha, alpha-gamma, gamma-gamma coupling in the ratio of 52:39:9); 4 = 5-phenyl-4-pentenenitrile; 5 = cinnamyl ether; 6 = cinnamyl alcohol; 7 = diethyl ester of cinnamyl malonate. b Each entry represents the average of at least three separate experiments. When cinnamyl bromide is reduced at 1.35 V in the presence of
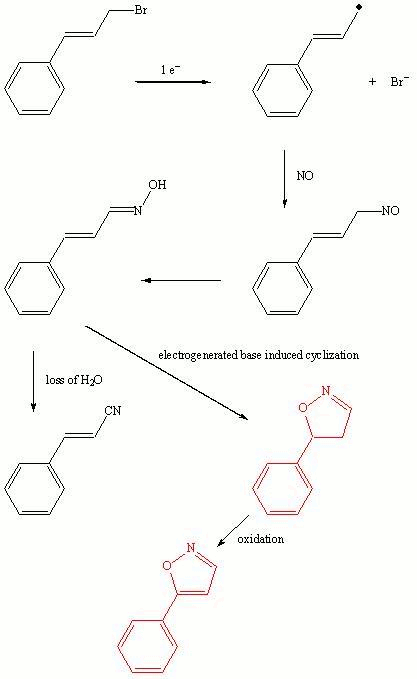
nitric oxide (NO), the electrogenerated cinnamyl radicals can efficiently couple with NO to give cinnamaldehyde oxime, cinnamonitrile, isoxazoline, isoxazole along with small amounts of allylbenzene,
beta-methylstyrene,
cinnamaldehyde, and benzonitrile.
There is no dimer observed and the proposed mechanism
is shown in Scheme 1. The heterocyclic products have diverse biological
activities including antifungal, antiviral, and anticancer properties, which
make this electrochemical process very appealing. Quantitation
of the products is being carried out and other allyl
halides will be examined for this reaction.
Scheme 13
Three
undergraduates and one graduate student participated in the research during the
past year. They have obtained extensive experience in various electrochemical
techniques, organic synthesis, and spectroscopic analysis. They have also
learned how to use internal standard method to quantitate
electrolysis products by using GC or GCMS. The research is inspiring for the
students to get more involved in chemistry studies. Two students received B.S.
degrees in the past year and one of them were admitted to graduate school (Drew
C. Brown M.S. program at The
PRF grant continues to help the principal investigator to gain experience in
undergraduate mentoring and maintain a productive research program at the early
stage of his career. It is expected that the research data collected during the
past year will enable the PI to submit several proposals to NSF, including one
for the CAREER award. There is no doubt that the PRF fund has made it possible
for the PI to initiate his research and put it on track in the academia.
References 1. 2. 3.