45978-AC1
Development of Organocatalysts for the Asymmetric Alkyne Addition to Aldehydes and Applications in Organic Synthesis
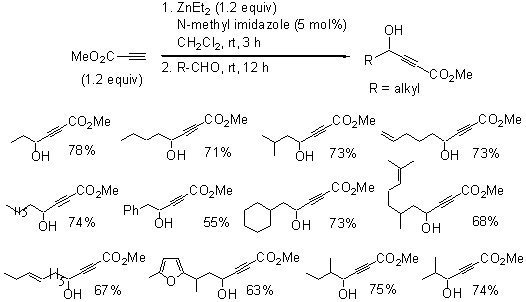
We have studied the catalytic alkyne addition to aldehydes for the synthesis of propargylic alcohols. Particularly, we have focused on the reaction of methyl propiolate with aldehydes to prepare g-hydroxy-a,b-acetylenic esters and the reactivity of the g-hydroxy-a,b-acetylenic esters. Previously, g-hydroxy-a,b-acetylenic esters were synthesized by treatment of methyl propiolate with nBuLi at extremely low temperature (-120 oC) followed by the addition of aldehydes. In 2006, we found that in the presence of 1,1'-bi-2-naphthol (BINOL), ZnEt2, Ti(OiPr)4 and HMPA, the reaction of methyl propiolate with aldehydes proceeded at room temperature with both good yields and high enantioselectivity. In 2007, You and coworkers used a similar procedure by replacing HMPA with a catalytic amount of N-methyl imidazole and found good yield and enantioselectivity for the reaction of methyl propiolate with benzaldehyde.
It is however not clear whether the use of the Lewis acid complex generated from BINOL and Ti(OiPr)4 is necessary when only the racemic products are desired. We have therefore tested the reaction of methyl propiolate with aldehydes in the presence of ZnEt2 and N-methyl imidazole without BINOL and Ti(OiPr)4 (Scheme 1). We find that N-methyl imidazole alone can activate ZnEt2 to deprotonate methyl propiolate to generate the corresponding alkynylzinc reagent. In addition, for the subsequent reaction with aldehydes, an additional Lewis acid catalyst like BINOL-Ti(OiPr)4 is not needed to activate the aldehydes, and the in situ-produced alkynylzinc reagent is nucleophilic enough for the addition. As shown in Scheme 1, using this method can give various alkyl substituted g-hydroxy-a,b-acetylenic esters under very mild conditions in good yields. Reactions were conducted at room temperature with the use of methyl propiolate (1.2 equiv), ZnEt2 (1.2 equiv), N-methylimidazole (5 mol%) and an aldehyde.
Scheme 1.
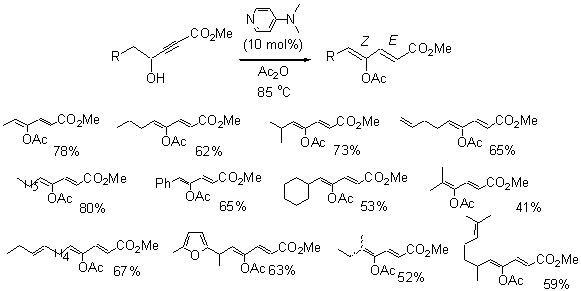
We have further studied the reaction of the g-hydroxy-a,b-acetyleneic esters in the presence of DMAP,
and found that when these compounds are treated with 10 mol% of DMAP in acetic
anhydride at 85 oC for 48 h, they have produced the novel g-acetoxy dienoates (Scheme 2). These results are summarized in Table 2. Generally, the reactions proceed smoothly to
give the g-acetoxy
dienoate products with good yields and high stereoselectivity. The sterically more hindered substrates such
as those shown in entries 11 and 12 normally give lower yields than the less
sterically demanding compounds. Scheme 3.
We have
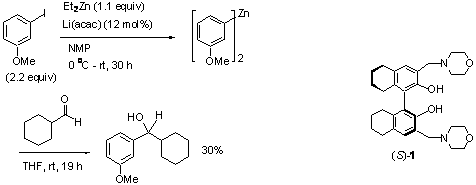
also studied the asymmetric arylzinc addition to aldehydes. Recently, Knochel reported a preparation of diarylzincs from
the reaction of aryliodides with a dialkylzinc under very mild conditions. The use of a dialkylzinc in this method
instead of an alkyllithium allows the preparation of a great variety of functional
arylzinc reagents. We tested the
reaction of aldehydes with a few functional arylzincs prepared in situ with this method, but only
observed very low yields of the alcohol products. Scheme 1 shows the reaction of m-iodoanisole with Et2Zn
under Knochel's conditions which presumably produced a diarylzinc complex. When this arylzinc was treated with
cyclohexanecarboxaldehyde at room temperature, the corresponding alcohol was
isolated in only 30% yield after 19 h. We tested the use of the chiral
ligand (S)-1 for the reaction of the functional arylzincs with
benzaldehyde. We are delighted to find
that in the presence of 10 mol% of (S)-1, the arylzinc generated in situ from m-iodoanisole reacts with cyclohexancarboxaldehyde to give the
desired secondary alcohol not only in greatly increased yield (94%) but also
with very high enantioselectivity (>99% ee).
Thus, (S)-1 not only activates the nucleophilic arylzinc addition to the
aldehyde but also has excellent stereocontrol.
High enantioselectivity has also been achieved when other aryl iodides
are used for the asymmetric addition to aldehydes. Scheme 3.