

46293-B1
Development of Synthetic Tools Using Ynol Ether Ionic Chemistry
Olefin attack towards cationic ketene ; formation of a,b-unsaturated ketone and unsaturated hemiketals
Our objective is to study the intramolecular reaction of homoallylic ynol ethers (2, see Scheme 1) with electrophiles in non-nucleophilic media. The purpose is to understand the reactivity of ynol ether derived cationic ketene intermediates (2) with strategically placed internal nucleophiles. This strategy should allow us to form hemiketal 5.
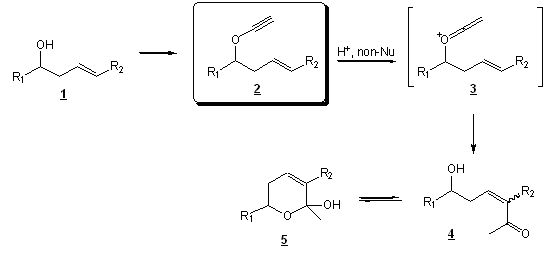
Scheme 1
In this first year of our project, we tried to synthesize two simple models : 2a (R1 = Ph, R2 = H) and 2b (R1 = H, R2 = Ph). In order to do so, we elected Greene's protocol (ref. 1), known to transform alcohols into ynol ethers. Homoallylic alcohols 1a (R1 = Ph, R2 = H) and 1b (R1 = H, R2 = Ph) should lead to 2a and 2b. Compound 1a is commercially available while 1b has to be prepared following Charette's protocol (ref. 2).
The formation of ynol ethers 2 following Greene's protocol (ref. 1) implies the reaction of alcohol 1 with KH and TCE (see Scheme 2) to form an intermediate : chloroenol ether 6 (usually not isolated). Treatment of this intermediate with 2 equivalents of BuLi should lead to the desired ynol ethers 2.
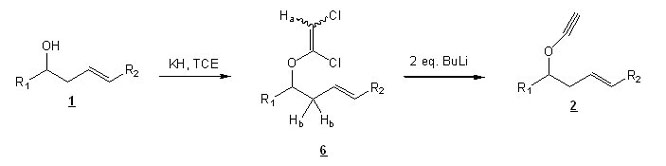
Scheme 2
Two talented undergraduate students (Katy Leduc and Gym Clerc Lentsolo Yalli) started the work on this project. They first synthesized compound 1b with comparable yields to those obtained in the litterature. Having 1a (commercially available) and 1b in hand, they tested the possibility of producing ynol ethers 2a and 2b. They meticulously followed Greene's protocol. Unfortunately, they could not obtained the desired product in both cases. In order to understand what went wrong, we decided to stop the reaction at the intermediate and isolate compound 6. We obtained this compound with yields reaching 80%. We then reacted 6 with BuLi and obtained the same results as above : no desired product. We then looked closely at our NMR spectra and GCMS analyses. We found that a lot of unsaturated compound 7 was formed.
How can this be formed ? This reaction usually works with alcohols, why does it fail here ? Well, Greene's protocol has been tested with a lot of alcohols, but not with homoallylic alcohols. We concluded that the problem was that BuLi, instead of abstracting Ha (that would lead to the desired product), abstracts allylic Hb and leads to the formation of 7 and ketene 8.
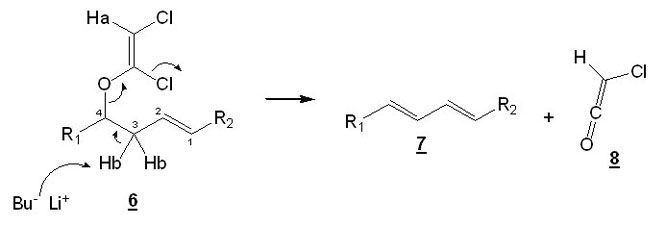
Scheme 3
We then redirect our research by trying to prepare homoallylic alcohols that do not possess any hydrogen at position 3 (no Hb). We elected alcohol 9. Using Fukumoto's protocol (ref. 3), Nelly Pons (summer 2008) undertook the synthesis of this product. The synthesis is almost completed, only one step remains to be done. We expect to complete this synthesis this fall (2008) with the help of another undergraduate student.
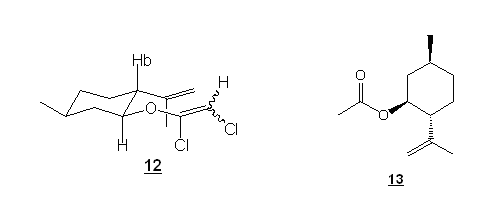
In the meantine, Jennyfer Mercklé undertook the synthesis of the ynol ether of commercially available isopulegol (10) (summer 2008). Isopulegol is a cyclic compound possessing the homoallylic alcohol functionality. We are confident that the synthesis of the ynol ether 11 should be possible, even if there is presence of one “dangerous” allylic hydrogen. Why ? No antiperiplanary elimination is possible with the chloroenol ether intermediate 12 (see below) in that particular case. Incidentally, when the alcohol 10 was subsequently treated with KH, TCE and BuLi, we were happy to observe that the ynol ether 11 can indeed be formed. However, some problems occured with the purification of this compound. Jennyfer worked a lot on this purification but summer came to an end and she had to leave our lab before she could fully optimize this purification.
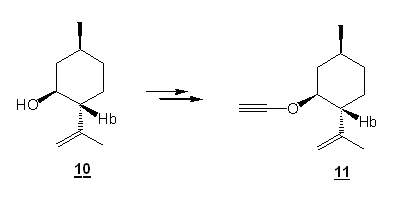
Scheme 4
However, since we were very eager to study our reaction, Jennyfer tested the reaction of this unpurified ynol ether with a solid acid, Montmorrolinite K10 (source of non-nucleophilic H+). The reaction was extremely rapid. After 5 minutes at 0°C, the starting product was completely consumed and one major compound was isolated. Spectra analysis revealed the structure of this compound : ynol ether hydrolysis product 13. We most probably formed the cationic ketene, but instead of being intramolecularly attacked by the alkene, it was attacked by a water molecule. We must repeat the experiment taking extreme care to exclude any water from the reaction mixture.
 References
References
1.
2.
3.