43108-B4
Catalytic Organic Molecular Imprints
Our research efforts have been aimed at the generation of organic, polymeric “molecular imprints”1 with improved—and novel—catalytic activities. Most recently, we have been attempting to generate an imprint with aldolase activity. Our system incorporates two features intended to enhance the catalytic activity of the imprints obtained: One, it employs a covalent bond between functionalized monomer and template; and two, it incorporates a nucleophile into the catalytic mechanism. Covalent imprinting allows for more precise positioning of the template (and thus of the analogous substrate) within the polymeric matrix, while direct participation of a catalytic nucleophilic functionality—a mechanistic feature that can lead to huge rate accelerations in intramolecular model systems—is expected to lead to enhanced rates.
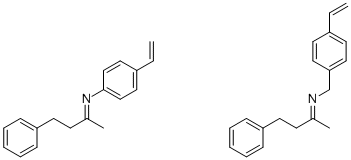
During the past few years, we have generated imprints against the following two imine templates (which were prepared from benzylacetone and either 4-vinylaniline or 4-vinylbenzylamine, respectively, in the presence of molecular sieves2 or magnesium perchlorate3 as a dehydrating agent):
Our hope was that after formation of the polymeric imprint,
hydrolysis of the imine linkage would yield an imprinted site with an amine
functionality poised to catalyze an aldol condensation (akin to the essential
participation of e-amino group of a
lysine residue in Class I aldolases).
However, under hydrolytic conditions benzylacetone was not released from
either of the imprints made with the above two imines.
Given that benzylacetone is an
aliphatic ketone, we have thus explored the formation of imprints with imine
templates formed from the aromatic ketone acetophenone and the aromatic ketone
2,2-dimethylpropiophenone, which also lacks α-protons. The Wulff group had shown that after
incorporation of the diimine between terephthalaldehyde and 4-vinylbenzylamine
into a molecular imprint, the terephthalaldehyde can be hydrolytically cleaved
in greater than 80% yield4—this system serves as our positive
control. In particular, we have
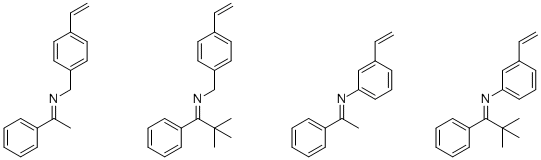
synthesized the following imines:
(We began employing 3-vinylaniline because it spontaneously
polymerizes far less readily than 4-vinylaniline.) We are in the process generating molecular
imprints with each of these imines (using the “recipe” employed by the Wulff
group4) and will then measure the extent of hydrolysis of each of the
incorporated imine functionalities.
Based upon the results obtained, we will design a functionalized imine
that we hope will ultimately generate imprints with aldolase activity.
1 Wulff, G. Angew. Chem. Int. Ed. Engl. 1995, 34, 1812–1832.
2 Kyba, E.P. Org.
Prep. Proc. 1970, 2, 149–156. 3 Chakraborti, A.K.; Bhagat, S.; Rudrawar, S. Tetrahedron Lett. 2004, 45, 7641–7644. 4 Wulff, G.; Heide, B.; Helfmeier, G. React. Polym. 1987, 6, 299–310.