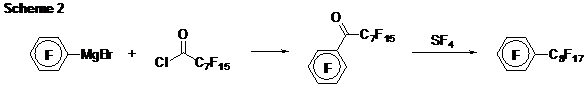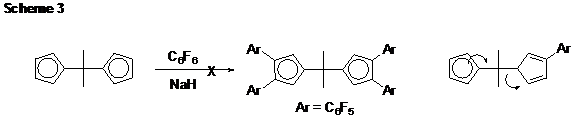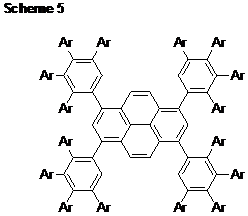ACS PRF | ACS
All e-Annual Reports

45698-AC7
Progress toward Highly Fluorinated Diels-Alder Polyphenylenes
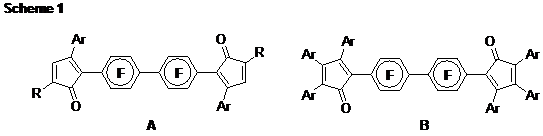
SEQ CHAPTER \h \r 1Part 1. Butylated Monomer Synthesis. About half of our synthetic work has focused on the biphenylene-linked bis(cyclopentadienone) (CPD) monomer system (A) as shown in Scheme 1. The bulky alkyl group (R = tert-butyl) allows attachment of only one additional substituent (Ar). This design keeps the synthesis simple (four steps) while allowing some modularity. We have been able to synthesize monomer A with Ar = C6F5, 4-C6F4CF3, and 4-C5F4N. All of the C-C bond forming steps are now achievable in high yields (> 75%) with one minor exception (attachment of C5F4N group, 50%).
Part 2. Perfluorinated Monomers. Our only successful perfluorinated monomer so far is B (Ar = 4-C6F4CF3) shown in Scheme 1. In the key step, the six aryl substituents are attached in one pot. The solvent we had been using (diglyme) was reacting under the harsh conditions. Through a lengthy process of trial-and-error, we found one substitute (HMPA), which gives 75% yield for the sequence.
Part 3. Cyclopentadiene Oxidation Chemistry. The last step in the synthesis of all our monomers is oxidation of a methylene group (CH2) to a carbonyl group (C=O). Our initial conditions (N,N-dimethyl-4-nitrosoaniline followed by acidic hydrolysis of the imine/nitrone) required forcing conditions to liberate the corresponding ketones. Yields were limited to about 30% after silica gel chromatography, an intolerable result. Going back to the drawing board (and to the literature), we found a copper catalyst that will enable many substituted cyclopentadienes to be oxidized to the corresponding cyclopentadienones by oxidation with O2 in yields exceeding 75%. Meanwhile B turns out to be made cleanly using a selenium dioxide oxidation (95%). Part 4. Ponytail Systems. Because our “workhorse” monomer system (A) is relatively low in fluorine content, we worried that progress toward “highly” fluorinated polyphenylenes might be limited only to the B monomer system. However, the modularity of A suggested an alternative approach. The student working on the perfluoro-4-tolyl-substituted monomer (Ar = 4-C6F4CF3) undertook the development of “ponytail” monomers in which the CF3 group was exchanged for a longer fluoroaliphatic chain. We now have a good synthesis of the required starting arene (Scheme 2). We are ready now to incorporate this interesting arene into our other monomer syntheses, both A and B types. Part 5. Dead Ends and Failures. In our proposal, we offered the an approach to an isopropylidene-linked monomer system (Scheme 3). However, these reactions fail because the isopropylidene linkage comes apart during the reaction! Careful observations and product analyses showed that 6,6-dimethylfulvene is formed, suggesting that the arylated cyclopentadiene is simply such a good leaving group (arrows in scheme) that the decomposition process becomes favorable and apparently more rapid even than deprotonation by sodium hydride. Another monomer system that we originally proposed is shown in Scheme 4. The whole synthetic sequence worked fine, except for one nasty surprise. The proposed trans isomer (shown below at left) was not obtained. Instead, the cis isomer formed exclusively, and upon oxidation to the diketone it underwent an intramolecular Diels-Alder reaction (at room temperature!) to form a complicated polycyclic species that we finally had to characterize by X-ray crystallography. This surprising self-Diels-Alder reaction has taught us something very important however: DDiels-Alder reactions between two CPD end groups should also be possible in monomer system A because of structural similarities, and we will need to keep a sharp eye out for this reaction as a possible cause of defects or low molecular weight. Part 6. Polymer and Solvents. At the close of the first fiscal year, we are not yet able to prepare high molecular weight polyphenylenes by combining our monomers (A, Ar = C6F5) with a dialkyne such as 1,4-diethynylbenzene. However, we now know that the monomers with Ar = 4-C6F4CF3 and Ar = 4-C5F4N react faster based on model reactions with phenylacetylene. We also have recently learned that the solvent of the model reactions with phenylacetylene can dramatically accelerate the reaction. Hydrogen-bonding solvents such as meta-cresol and 2,2,2-trifluoroethanol seem to work best. We think the CPD carbonyl oxygens are accepting hydrogen bonding from these solvents, increasing their electrophilicity and accelerating the “inverse electron demand” Diels Alder reactions. We hope to use this new knowledge to make progress toward these polymers in the coming year. Part 7. Fluorinated Nanodentritic Structures. With funding from the Summer Faculty Fellowship program, my colleague and his student made progress toward the synthesis of the species shown in Scheme 5 (Ar = C6F5). Work is underway to complete this synthesis and explore its behavior in fluorous and non-fluorous solvents.