
ACS PRF | ACS
All e-Annual Reports

41461-B4
Green Chemistry: Diels-Alder Reactions in High Temperature Water
Investigating Diels-Alder, DA, reactions in high temperature water (HTW, T > 100 oC, P> 1 bar) provides the opportunity to gain mechanistic insights to the aqueous solvent acceleration of these reactions, while creating an important green solvent replacement technology that can increase safety and reduce costs of chemical production. HTW is a remarkable solvent. At high temperatures and pressures, the physical properties of water vary dramatically from those found at ambient conditions. Specifically, the dielectric constant (e) of water decreases from e = 80 at STP, to e = 31 at 225 °C, P = 100 bar, and finally to e = 6 at the critical point (Tc = 374 °C, Pc = 221) due to the steady decrease in the effectiveness of hydrogen bonding with increasing temperature. This allows the solubility of non-polar organics to be tuned using the temperature and pressure.
We have been working to develop the N-ethylmaleiminde and 9-methylanthracene reaction system. The 9-methylanthracene is very stable under HWT conditions while the N-ethylmaleimide is less so. We have confirmed over 50% reaction of the 9-methylanthracene with N-ethylmaleiminde to the DA coupled product (HPLC and GC-MS) at 106 o C with a reaction time of 20 min. However, ~30% of the 9-methylanthracene remained was unaccounted for. We believe that the DA reaction rate is actually very high, possibly close to 100% conversion, unfortunately yielding a thermally unstable product that reactant further to unknown materials. Two undergraduate students worked on this project in the last reporting cycle. They presented their results at the National American Chemical Society meeting in 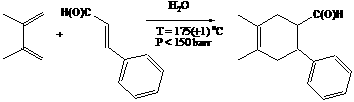 We have demonstrated that basic DA reactions are possible at unexpectedly low temperatures in HTW (T =175 oC, P>150 bar). Our high-pressure view cell allows for confirmation of the homogeneous condensed state - a single aqueous phase without separate gaseous or organic layers. We alternate this cell with a second rector system, an opaque 25 mL reactor. The reaction of 2,3-dimethyl-1,3-butadiene and trans-cinnamaldehyde, cinnamonitrile, or cinnamyl alcohol were run using a 10:1 ratio of the diene and dienophile, respectively at 175o C, and ~200 bar for 1 hour. The total organics in the system was less than 5% of the total reactor volume. Analysis by GC-MS indicated a clean conversion to the DA coupled product. We are developing the quantitization methods. Importantly, this result stands in contrast to previous reports of DA reactions in HTW where no coupling product was seen below 280 oC. We will utilize this system to examine the components of DA acceleration mechanism, hydrophobic acceleration and H-bonding. Altering substituents on the vinyl group will allow us to investigate the effects of electron withdrawing and electron donating groups. Additionally, changing substitutents on the aromatic ring will allow us a second point to manipulate the reactions.
We have demonstrated that basic DA reactions are possible at unexpectedly low temperatures in HTW (T =175 oC, P>150 bar). Our high-pressure view cell allows for confirmation of the homogeneous condensed state - a single aqueous phase without separate gaseous or organic layers. We alternate this cell with a second rector system, an opaque 25 mL reactor. The reaction of 2,3-dimethyl-1,3-butadiene and trans-cinnamaldehyde, cinnamonitrile, or cinnamyl alcohol were run using a 10:1 ratio of the diene and dienophile, respectively at 175o C, and ~200 bar for 1 hour. The total organics in the system was less than 5% of the total reactor volume. Analysis by GC-MS indicated a clean conversion to the DA coupled product. We are developing the quantitization methods. Importantly, this result stands in contrast to previous reports of DA reactions in HTW where no coupling product was seen below 280 oC. We will utilize this system to examine the components of DA acceleration mechanism, hydrophobic acceleration and H-bonding. Altering substituents on the vinyl group will allow us to investigate the effects of electron withdrawing and electron donating groups. Additionally, changing substitutents on the aromatic ring will allow us a second point to manipulate the reactions.