ACS PRF | ACS
All e-Annual Reports

43451-AC3
New Directions in Iron-Cyanide Chemistry
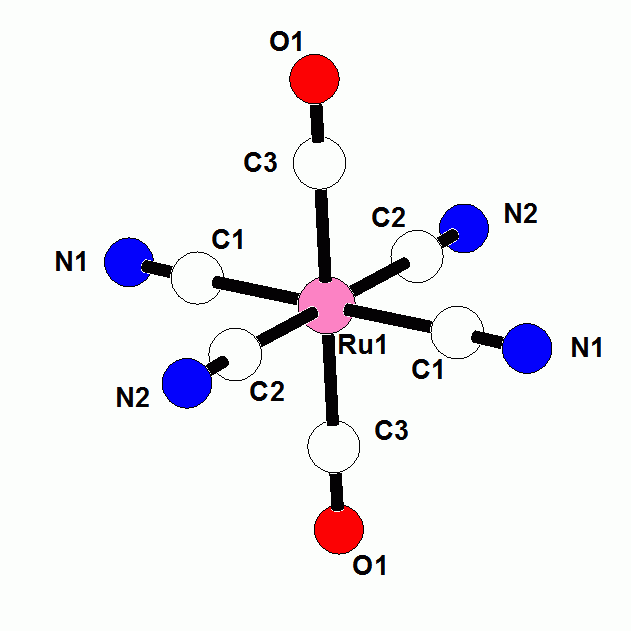
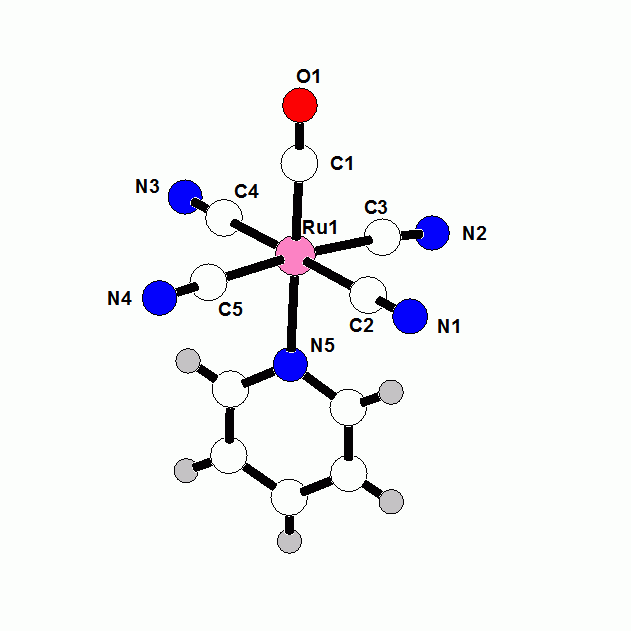
Fe(CN)(CO) coordination is a feature of active sites of [Ni-Fe] and [Fe-Fe] hydrogenase enzymes. Using these enzymes, microorganism evolved the capability of catalyzing the reaction (2H+ + 2e- = H2). The Pt electrode is the only other catalyst for this reaction. Fuel cell are now completely dependent on Pt electrodes. The fundamental information that we are learning about Fe-CN coordination compounds should provide insight into the mechanism of H2 production and H2 activation by these hydrogenase enzymes. In this grant year, we extended our studies to Ru and Os cyanide complexes. The chemistry of Ru and Os cyanide compounds is even less developed than that of Fe. There are only a handful of [M(CN)xLy] compounds of Ru and Os where x is less than five and L are monodentate ligands. The direct reaction of CN- with Ru and Os halides has proved to be more complicated than the simple reactions of CN- with FeCl2 and FeCl3 that we have developed. We have been more successful using a synthetic route that we developed in Fe-CN chemistry. Ferrocyanide salts such as K4[FeII(CN)6] are extremely inert to substitution reactions such that ferrocyanide salts are actually approved food additives. We discovered that there is a dramatic increase in the reactivity of [R4N]4[FeII(CN)6] salts in organic solvents with respect to ligand substitution reactions. Previous studies were limited to aqueous solvents because the previously known salts were not soluble in organic solvents. We have found similar increases lability of the CN- ligands in [R4E]4[MII(CN6)] salts of Ru and Os in non-aqueous solvents. Reactions with CO at atmospheric temperature gives stepwise production of the new compounds [RuII(CN)5(CO)]2- (Figure 1) and trans-[RuII(CN)4(CO)2]2- (Figure 2). The iron analog of the former compound has been known for more than 120 years. The reaction of [RuII(CN)5(CO)]2- in pyridine gives trans-[RuII(CN)4(py)(CO)]2- (Figure 3). The corresponding Os complexes are also being structurally and spectroscopically characterized Fig. 1 [RuII(CN)5(CO)]2- Fig. 2 trans-[RuII(CN)4(CO)2]2- Fig. 3 trans-[RuII(CN)4(py)(CO)]2- The periodic trends between analogous Fe, Ru and Os cyanide compounds are being evaluated. A current objective is the generation of metal hydride and metal H2 compounds which may be a type of intermediate which may exist in the hydrogenase enzymes.