ACS PRF | ACS
All e-Annual Reports

44037-AC3
Self-Assembly of Metallic Nanoparticles Mediated by Metal-Organic and Metal-Organometallic Coordination Networks
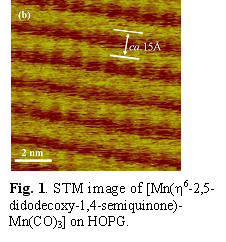 Orgaometallic pi-complexes such as [(h6-2,5-didodecoxy-1,4-semiquinone)Mn(CO)3] assemble on HOPG surfaces by forming a strongly hydrogen-bonded quinonoid framework to form ridges that are connected by overlapping hydrophobic arms of the didocoxy groups. (Fig. 1). These robust 2D systems involve metal-ligand coordination, hydrogenation bonding and hydrophobic interdigitation. Self assembly of this sort is unique and my have catalytic applications in view of the facile protonation/deprotonation of the quinonoid moiety. Calculations support the expectation that the semiquinone ring is attracted to the HOPG, with the carbonyl ligands projecting up away from the surface.
Orgaometallic pi-complexes such as [(h6-2,5-didodecoxy-1,4-semiquinone)Mn(CO)3] assemble on HOPG surfaces by forming a strongly hydrogen-bonded quinonoid framework to form ridges that are connected by overlapping hydrophobic arms of the didocoxy groups. (Fig. 1). These robust 2D systems involve metal-ligand coordination, hydrogenation bonding and hydrophobic interdigitation. Self assembly of this sort is unique and my have catalytic applications in view of the facile protonation/deprotonation of the quinonoid moiety. Calculations support the expectation that the semiquinone ring is attracted to the HOPG, with the carbonyl ligands projecting up away from the surface.
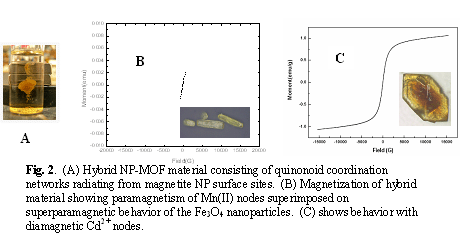
Magnetic nanoparticles can be surface modified with Organometallic quinonoid complexes of Mn, Rh, and Ru. Scheme 1 shows the behavior with FePt NPs, with similar behavior obtaining with Fe3O4 NPs. Once the quinonoid organometallics are anchored on the nanoparticle surfaces, the addition of suitable reagents leads to crystalline Metal Organic Framework material. This results in stable MOF materials that incorporate minute quantities of superparamagnetic nanoparticle. This allows for magnetic separation after the materials are sy6nthesized and possible utilized in catalysis.
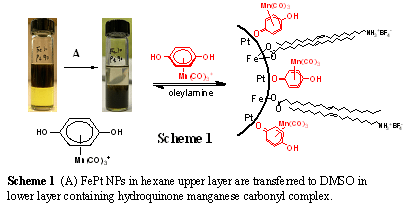 |
Figure 2 shows the new materials attracted to a permanent magnet. The magnetization curves demonstrate that the percentage of metallic NP is low, but high enough to allow magnetic decantation. Exciting uses of materials such as these, especially ones containing the active transition metals Rh and Ru appear feasible (se references), such as carbon –carbon coupling and ring opening reactions.