ACS PRF | ACS
All e-Annual Reports

42634-AC5
Novel Aluminosilicate Solid Acid Catalysts Using Building Block Strategies
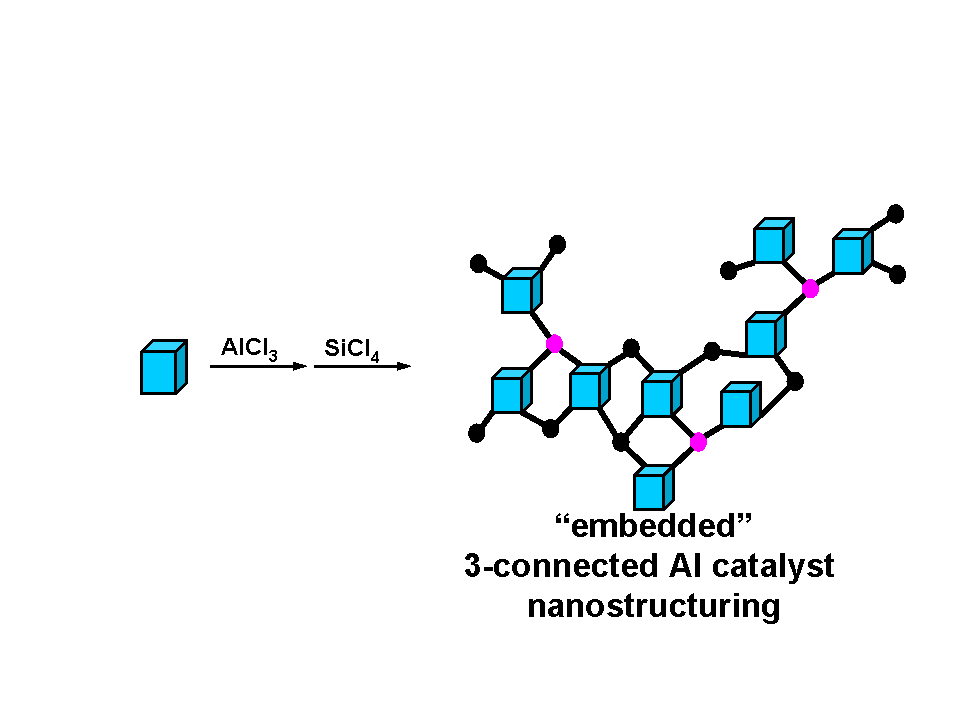
We proposed to synthesize and characterize a family of novel aluminosilicate materials that are prepared via a new nonaqueous building block methodology recently developed our laboratories. The essence of this approach is illustrated below in Figure 1 where aluminum trichloride is reacted with a trialkyltin functionalized, cubic spherosilicate, Si8O20(SnR3)8 in aprotic solvents to yield building block matrix cross linked together with aluminum ions. By adjusting the stoichiometric ratio of aluminum to Si8O20 cube atomically dispersed “embedded” aluminum centers may be obtained. By embedded we mean that the maximum connectivity (cross linking) of aluminum to individual cubes has been attained, in this case 3-connected Al centers. The nominal three-coordination expected around aluminum is quite unique for alumino silicates and is not attainable via traditional hydrothermal or sol-gel syntheses. It is from this starting point that we have begun to study the acid-base properties of these aluminosilicate solids. The notion that 3-coordinate aluminum centers are formed as cross linking of cubes occurs is of interest because of the propensity for these potent Lewis acid centers to react and coordinate donating species. However, the nonpolar, aprotic media used in the synthesis (toluene, methylene chloride, ether) do not allow one to easily identify potential donors. Furthermore, gravimetric analysis of the extent of cross linking is inconsistent with coordination of solvent in all cases thus far. Our current efforts to characterize these interesting Lewis acids are focused in two directions. First, we have devised a simple plan to produce soluble molecular analogues to the solid matrix shown above by utilizing POSS substitutes for the spherosilicate building block. Isobutyl substituted POSS starting materials can easily be transformed to the mono trimethyltin derivative and then reacted with AlCl3 to produce the POSS trimer. This soluble analogue will be characterized in a number of ways (X-ray diffraction, IR, NMR, MALDI MS). Second, the reactions of the aluminum in these molecules will be of particular interest within the context of the work supported by the PRF. Reaction of the Lewis and Brønsted sites in aluminosilicates with pyridine is reported to give rise to a set of distinctive vibrational signatures for each site. In our case, we should have only Lewis acid sites and thus expect to observe only one set of bands. If, however, these Lewis acid sites are first reacted with a protic substrate such as an alcohol, a 4-coordinate Brønsted acid site will be formed. Subsequent reaction with pyridine should then lead to the IR signature consistent with this type of site instead of the Lewis site that it started as. Reactivity studies of these aluminosilicate materials have already begun in the context of a collaborative project with Dr. James Goodwin in the chemical engineering department of Clemson University. We have begun to evaluate the potential of the solid acid catalysts in the transesterification of triglycerides to biodiesel. The potential advantage that these new solid acid catalysts have over zeolitic solids is the accessibility of large triglyceride molecules (biological fats and oils contain ~C18 chains) to the acid sites. The building block materials that we prepared have much larger pores than microporous zeolites. The results of initial studies indicate taht even at this the preliminary stage of our work, the titanium building block solid acids are as effective as traditional solid acids. Future work on this project will focus on 1) precise characterization of the aluminum sites in these matrices and 2) surveying their reactivity in solid acid catalyzed reactions such as transesterification and olefin polymerization reactions.