
ACS PRF | ACS
All e-Annual Reports

42425-AC4
Examination of the Mechanisms of the Schmidt and Modified-Schmidt Reactions
A common method for synthesizing amides is the Schmidt reaction between hydrazoic acid and ketones. The utility of the Schmidt reaction was long thought to be limited to the use of hydrazoic acid, as early work on the Schmidt reaction showed that no product or very little product was obtained when alkyl azides were employed. This observation seems to indicate that the parent reaction follows path b instead of path a (Figure 1). Path b would not be possible for alkyl azides as this would require loss of the alkyl group as a carbocation. However, in the early
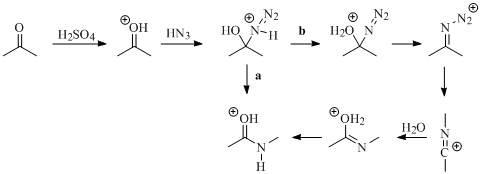
Figure 1. Possible mechanisms for the Schmidt reaction between acetone and hydrazoic acid.
1990's, an intramolecular version of this reaction was found to give high yields with the use of an appropriate Lewis acid. Presumably, this reaction goes through path a. It is unclear why having the alkyl group intramolecular should now make path a more likely. Another possibility is that alkyl azides are not nucleophilic enough to attack the activated carbonyl, but the more favorable entropy of the intramolecular attack lowers the free energy enough to make this reaction feasible.
We are using ab initio methods to calculate the mechanisms of these various reactions in order to understand the above reactivity patterns. So far, our preliminary results have provided more questions than answers. Results for initial calculations on an intramolecular and intermolecular system are given in Figure 2. For both reactions, the azide attack energy barrier (uncorrected for zpe or temperature) is 5.7 kcal/mol. Thus, whether or not the reaction is
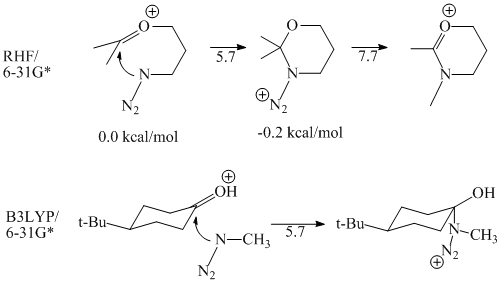
Figure 2. Results for an intramolecular and intermolecular version of the alkyl Schmidt reaction.
intramolecular does not seem to affect the energy of attack. In addition, the small height of the barrier seems to disprove the assumption that alkyl azides are not nucleophilic enough to attack the carbon of the carbonyl. The barrier for migration in the intramolecular case was 7.9 kcal/mol.
More complete calculations on an intramolecular reaction (Figure 3) give a 0.4 kcal/mol energy barrier for azide attack and 8.3 kcal/mol for migration. The corresponding calculations on an alkyl azide intermolecular reaction give an azide attack barrier of 0.8 kcal/mol and a migration barrier of 11 kcal/mol (Figure 4). Again, intermolecular versus intramolecular does not seem to have an effect on the energetics. Presumably, when the entropy is included the intermolecular reaction will have a higher barrier than the intramolecular version. However, the barrier is so small that it seems unlikely that the entropy will be large enough to make the intermolecular reaction energetically unfeasible.
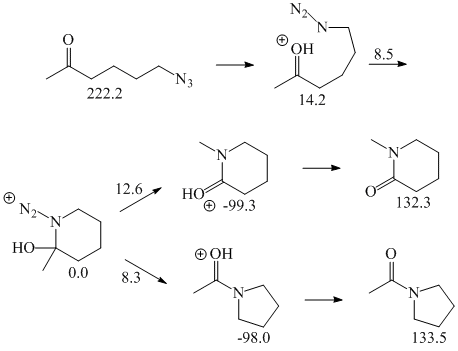
Figure 3. RHF/6-31G* Energies for the intramolecular Schmidt reaction of 6-azido-2-hexanone.

Figure 4. RHF/6-31G* Energies for the Schmidt reaction between acetone and ethyl azide.
Our first task is to convert our energies into free energies. This will provide a more complete picture of the effect of entropy. Secondly, a higher level of theory may give significantly different results. All of the intermediates and most of the transition states have already been calculated at the MP2/6-31G* level of theory. We are currently working on locating the last couple of transition states. Lastly, solvent could play a significant role in the reactivities of these compounds. We intend to add both explicit and implicit solvent to our calculations. The IEFPCM algorithm in Gaussian03 will be used to add implicit solvent. In addition, one to three water molecules will be added to each calculation to account for hydrogen bonds, but the bulk solvent will still be modeled implicitly. We will also continue our calculations on the parent reaction between hydrazoic acid and acetone for comparison to the alkyl azide reactions.