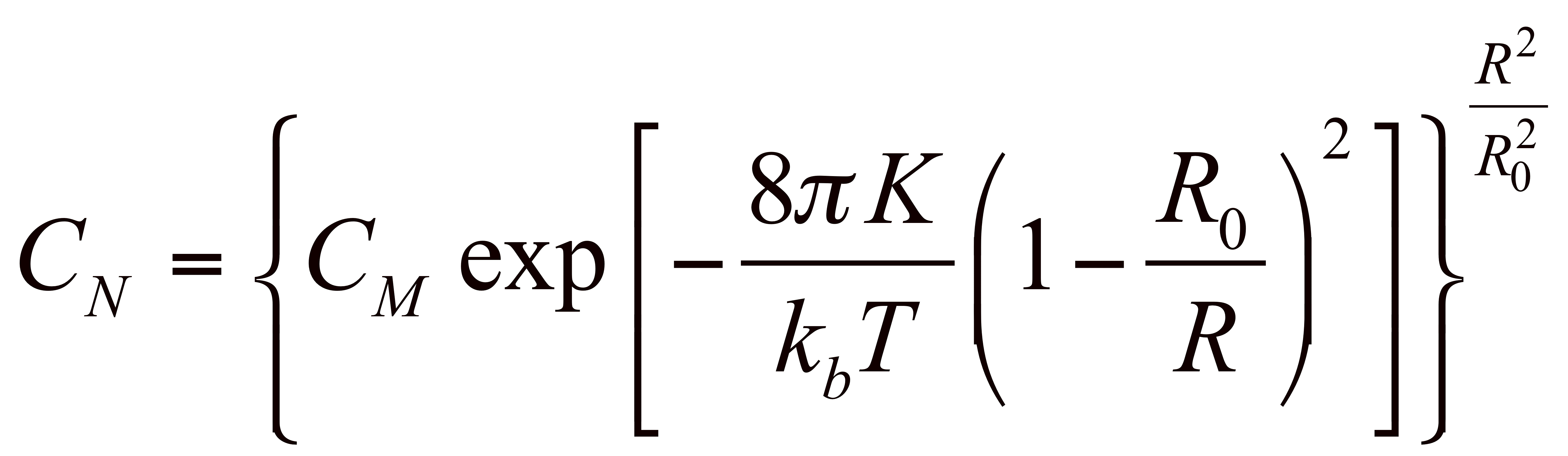ACS PRF | ACS
All e-Annual Reports

41016-AC7
Electron Microscopy Characterization of Size Distributions of Surfactant Microstructures
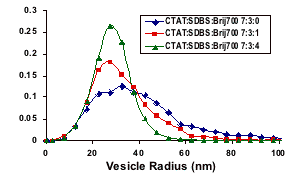
The formation of spontaneous surfactant bilayer vesicles is observed in a few chemical systems, most widely in mixtures of cationic and anionic surfactants. While PEG-lipids have been studied as stabilizers of rigid phospholipid vesicles (in applications including drug delivery), the effect of grafted polymers on the micro-mechanical properties of a thermally fluctuating vesicle membrane is not well understood either theoretically or experimentally. Here we use a variety of tethered polymers to tailor the bilayer properties of membranes, including the rigidity and spontaneous curvature, and are able to create extremely monodisperse vesicles. Using dynamic light scattering (DLS) we are able to obtain an average diameter and polydispersity and from cryogenic transmission electron microscopy (cryo-TEM) we can obtain membrane rigidity and spontaneous curvature from the exact size distribution of the vesicles. Thus, by direct experimental observation of the spontaneous vesicle size distribution as a function of PEG-lipids, Figure 1: The partial phase diagram for CTAT/SDBS/Brij-700 system in water. Phase symbols are as follows: M=spherical micelle, R=viscous rod-like micelles, X= unresolved multiphase, and V= vesicular structures. The dotted regions inside the vesicle phase are regions with monodisperse vesicles. With no Brij present, no vesicles form at equimolar CTAT/SDBS concentrations. Adding polymer surfactants such as Brij-700 (polyethylene oxide surfactants series) that can tether to the bilayer to the well studied catanionic vesicle system of cetyltrimethylammonium tosylate (CTAT) and sodium dodecylbenzene sulfonate (SDBS) strongly influences the size distribution of the spontaneous vesicles as shown in Fig. 2. A combination of entropy and bending energy determines the size distribution of the vesicles which can be fit to the measured size distribution to determine the bending elastic constants, K = kappa + kappabar/2, and the spontaneous radius of curvature, R0: The mean of the distribution is set by the spontaneous curvature radius, R0 while the width of the distribution is proportional to the sum of the bending and Gaussian elastic constants: K=kappa + kappabar/2. Adding the polymers increases the bending constant by more than a factor of 3, as is expected from theory – the practical effect is to make the spontaneous vesicles nearly monodisperse. There is a direct correlation between adding the tethered polymer and increasing the bilayer rigidity, which is independent of the details of the polymer length or chemistry as shown in Table 2. As the rigidity increases there is a higher energy penalty for deviations from the spontaneous curvature leading to more monodisperse size distributions. Also the spontaneous curvature decreases as more tethered polymer is added to the system as expected since a higher curvature is induced to accommodate the polymer, which suggests a greater segregation of the polymer in the bilayer. Figure 2: Summary of microscopy results for CTAT:SDBS:Brij700 vesicles at 1% in water. ABOVE: Comparison of cryo-TEM vesicle size histograms for various levels of Brij700, namely 0, 0.8, and 3 mol%, showing a progressive narrowing of the size distribution. The average radius as found by FF-TEM is in good agreement with the values of the hydrodynamic radius as found by DLS. The values found from DLS were always larger since scattering provides a mass-averaged size estimate as opposed to the number-average estimate available from microscopy. Surfactant <R> (nm) <r2> σ σ/<R> K (kBT) DDLS (nm) Brij-30 38.1 1006.4 7.4 0.19 0.53 85.3 Brij-35 46.8 1555.6 12.0 0.26 0.30 99.1 Brij-76 27.2 497.5 2.5 0.09 2.4 62.0 Brij-78 25.1 431.3 4.4 0.18 0.65 60.8 Brij-97 31.8 695.0 5.3 0.17 0.72 43.1 Brij-98 30.2 634.4 6.1 0.20 0.49 55.3 Brij-700 32.2 712.2 5.4 0.17 0.72 71.0 Table 2: Values for the average radius, <R>, spread of the distribution, σ/<R>, and rigidity, K, as found by TEM and the hydrodynamic diameter, DDLS, found by DLS for various non-ionic surfactants mixed as CTAT/SDBS/PEO 7:3:4wt. For CTAT/SDBS vesicles with no polymer, K=0.15 kT. In summary, cryo-electron, freeze-fracture TEM and dynamic light scattering show that tethered polymers increase the bending elastic constants of spontaneous vesicles, thereby sharpening the size distribution. It is relatively simple to make nearly monodisperse, spontaneous vesicles by adding a variety of simple tethered polymers to existing spontaneous vesicle formulations. Such monodisperse vesicles should be beneficial as templates for polymerization or other chemical processes and the general principles of polymer tethering increasing the spontaneous curvature and the bending elasticity should be easily transferred to biologically relevant lipid systems.  we can better understand the effect of grafted polymers on fluctuating membranes, assisting in the development of new nano-structured materials.
we can better understand the effect of grafted polymers on fluctuating membranes, assisting in the development of new nano-structured materials.