ACS PRF | ACS
All e-Annual Reports

42476-GB1
Development and Stereochemical Investigation of the First Microwave-Assisted Aza-Cope Rearrangement-Mannich Cyclization
Previously we detailed the development of a microwave-assisted aza-Cope rearrangementóMannich cyclization [1] and a microwave-assisted epoxide aminolysis to prepare amino alcohols used as aza-CopeóMannich substrates [2].† In the past year, we have explored the microwave-assisted aza-CopeóMannich reaction in more detail, and are using it and the microwave-assisted aminolysis to assemble a pyrrolizidine alkaloid core that is common to a number of biologically interesting natural products.† ††
I.† Microwave-assisted aza-Cope rearrangementóMannich cyclization
In previous reports, we described the effect of the size of the amine protecting group on diastereoselectivity in the microwave-assisted aza-CopeóMannich reaction.† We also demonstrated that diastereoselectivity could be improved by lowering reaction temperature.† Since then, we have examined the effects of additional variables on the yield and diastereoselectivity of the reaction.† Selected examples from these investigations are given in the Table.
The type of desiccant was found to have no significant effect on diastereoselectivity or yield (entries 1 and 3), but in the absence of desiccant, diastereoselectivity declined (entry 6).† Choice of solvent was found to affect yield.† Toluene and acetonitrile (entries 5 and 3) produced similar results, but for microwave-assisted aza-CopeóMannich reactions were performed in methanol, yields were clearly inferior (entry 4).†
The aza-CopeóMannich reaction was also conducted at room temperature to compare diastereoselectivity and yield to that obtained via microwave heating (entry 2).† Conversion to products was maximized after 144 hours, and improved diastereoselectivity was observed.† However, the lengthy reaction time makes the microwave-assisted version more attractive in spite of its slightly lower diastereoselectivity.
Table.
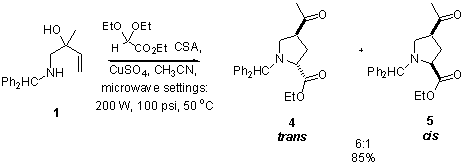
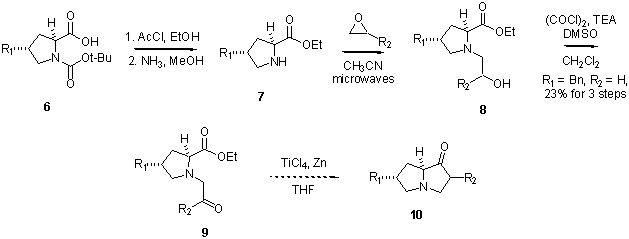
In addition, we have demonstrated the versatility of this reaction by using ethyl glyoxylate diethyl acetal as the aldehyde in this reaction.† Good diastereoselectivities and yields were observed (Scheme 1) [1]. Scheme 1 Finally, we anticipate using pyrrolidines such as 4 as substrates in a microwave-assisted aminolysis [2] to generate amino alcohol 8 (Scheme 2).† Following oxidation of alcohol 8, ketone 9 can be used as a substrate in a reductive coupling reaction to form pyrrolizidinone 10.† In a model system, amino ketone 9 has been generated in 23% yield from boc-proline 6 (R1 = H). Scheme 2 References (1) Johnson, B. F.; (2) Desai, H.; D'Souza, B. R.; Foether, D.; Johnson, B. F.; Lindsay, H. A. Synthesis 2007, 902.