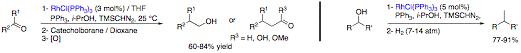ACS PRF | ACS
All e-Annual Reports

43579-AC1
A New Paradigm from Nature Using Transition Metal Complexes: Multi-Catalytic and Cascade Processes toward the Synthesis of Organic Molecules
This proposal was aimed at the development of new methodologies for C-C bond formation based on multicatalytic and cascade processes using transition metal complexes. Serious progresses have been made toward this goal. First of all, we have recently disclosed that N-heterocyclic carbene copper complexes and copper (I) salts efficiently catalyzed the methylenation of a variety of aliphatic and aromatic aldehydes and ketones in the presence of trimethylsilyldiazomethane, triphenylphosphine and 2-propanol. The copper catalysts are not only inexpensive compared to rhodium complexes (RhCl(PPh3)3, previously used to catalyze this reaction) but they also exhibit better functional group compatibility.
This important discovery provides a new tool to efficiently perform methylenation reaction of complex substrates. For instance, tricarbonyl 1 (the requisite precursor for the ring closing metathesis to lead to (+)-axenone) is sensitive to competitive retro-Michael reaction under the standard rhodium-catalyzed methylenation reaction. We are currently studying the copper-catalyzed methylenation reaction to achieve this task. Similarly, this strategy will be used for the total syntheses of (-)-isotalicene and (+)-isoitalicene.
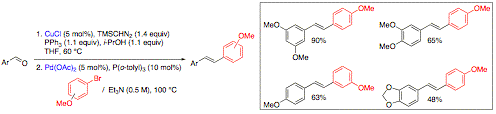
The total synthesis of Hodgsonox, is also underway. The synthetic strategy involves the formation of the 5-membered ring through a methylenation-ring closing metathesis and cyclization to form the pyran via O-H insertion. So far, we have prepared large amount of chiral enantiopur alcohol 4 and elaborated the lactone 3 through an enantioselective reduction of the corresponding b-ceto lactone. The synthesis of the requisite precursor 2 is currently undergoing. We have also developed a novel multicatalytic strategy to prepare E-stilbenes directly from benzyl alcohols and/or benzaldehyde substrates to avoid the need for the isolation of the styrene intermediate. Indeed, we have prepared stilbenoid derivatives with moderate to high yields from benzaldehydes substrate (without isolating the styrene intermediate) by performing a copper-catalyzed methylenation-palladium-catalyzed Heck cross-coupling multicatalytic cascade process. In particular, we have prepared resveratrol trimethylether in 90% yield via this one pot procedure. This yield compares favorably with those obtained in the literature. This work has been completed and included in a full paper published in Journal of American Chemical Society (JACS 2007, 129, 13321-13326). Following our work on rhodium-catalyzed cascade reactions, a methylenation-hydroboration homologative process, we have also completed and published the reductive one-carbon homologation methodology of aldehydes and ketones. Furthermore, we have also studied the combination of the rhodium-catalyzed methylenation-hydroboration cascade with a palladium-catalyzed cross-coupling reaction. One of the key points was to find the suitable hydroborating agent to generate the requisite aliphatic organoborane for the Suzuki cross-coupling reaction. Our initial results showed that it was not possible to use an organoborane derived from catecholborane, the hydroborating agent initially used in the rhodium-catalyzed methylenation-hydroboration cascade. Indeed, to perform Suzuki couplings with an aliphatic organoborane, 9-borabicyclononane (9-BBN) was required as the hydroborating reagent. Preliminary studies were performed with Garner's aldehyde as the substrate. Following the rhodium-catalyzed methylenation reaction, a solution of 9-BBN in toluene was added and after heating at 80 °C, the reaction mixture was submitted to the palladium-catalyzed Suzuki coupling using palladium tetrakistriphenylphosphine as catalyst and a variety of electrophiles. Good yields of 43-75% were obtained for this sequence of reactions, in which none of the intermediates were isolated. This process allowed the direct transformation of an aldehyde to an alkane via the formation of a carbon-carbon bond. Furthermore, the methylenation can also be performed using copper iodide or chloride in THF and dioxane, respectively, in high yields. The overall yields for the one-pot multicatalytic process is typically higher when using copper catalysts instead of the Wilkinson's complex, as copper salts are known to aid cross-coupling reactions. We performed the total synthesis of homophenylalanine hydrochloride from Garner's aldehyde with an overall yield of 55% (lit. Yield: 46%). We have extended this multicatalytic process to other aldehydes as well. This work has been also published (JACS 2007, 129, 13321-13326). Overall this grant has led to the development of new multicalytic processes as anticipated which has been published in the most prestigious journal in chemistry. Current efforts are toward the synthesis of natural molecules using these processes.