ACS PRF | ACS
All e-Annual Reports

42953-AC5
Dynamic Electrostatic Self-Assembly of Nanoparticle Superlattices
The financial support from the ACS Petroleum Research Find (under Award #42953) allowed us to achieve most objectives outlined in the original Proposal on nanoscale electrostatic self-assembly (ESA, Fig. 1). The research we pursued under the auspices of the Grant resulted in several publications in top quality journals including Science, Nano Letters and JACS, an in several press releases in popular science and opinion media (e.g., Chemical & Engineering News, Chicago Tribune). The Award was of immense help in initiating nanoscale research in my group and training students in nanoscale synthesis and related instrumental/characterization techniques.
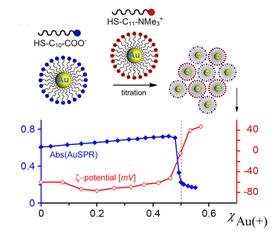
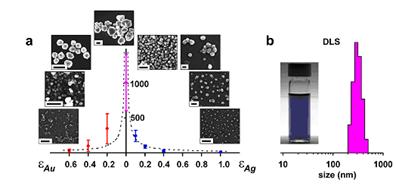
In the first year of this Project, we have been able to demonstrate efficient self-assembly of nanoscopic components into large, three-dimensional structures using electrostatic interactions. The first paper on this topic was published in Science (312, 420, 2006) and described how the electrostatic forces can mediate formation of unusual, diamond-like crystals from charged, spherical nanoparticles, NPs (Fig. 2, top). In the follow up work published in Nano Letters (6, 1896, 2006) we have shown how ESA based on metallic nanoparticles can give rise to nanostructures of unusual optical properties. Here, we combined experiments with high-end electrodynamics simulations to explain the non-additive nature of optical spectra of assemblies composed of two types of nanoscopic building blocks. Fig. 2 Equilibrium and non-equilibrium ESA. (top) Supracrystals formed from oppositely charged nanoparticles (bottom) Light reversible crystals and toroids whose ESA is mediated by photoinduced electric dipoles. Year 2. In an effort prepare better quality building blocks for nanoscale ESA, we developed a titration method that reports the amount of charge tethered onto the NPs with truly unprecedented accuracy. This method is based on our discovery that oppositely charged NPs remain stable in solution until reaching certain critical composition. Importantly, we found that this composition corresponds to a point where the charges on positively and negatively charged NPs are balanced (Fig. 3). In this way, using NP “standards” of known charge (either positive or negative), we were able to measure – from the location of the precipitation point – the unknown charges on the particles of interest. These measurements reported charge with precision of 2-3% which had previously been well beyond the reach of any other analytical method. We published these exciting results as a communication in JACS (128, 15046, 2006). Fig. 3. (a) Scheme of the titration experiment in which a solution of negatively charged NPs (e.g., Au/SH-(CH2)11-COO-) is titrated with a solution containing positively charged NP “standards” (e.g., Ag/SH-(CH2)11-N(CH3)3+). The titrated nanoparticles precipitate from solution only upon reaching the point of NP charge neutrality. (b) Intensity of the Au SPR band at lmax 520-550 nm (blue line) and the values of the z-potential (red line) for the titrations of oppositely charged, 5.5 nm AuNPs. Having perfected the preparation of ESA components and the methods of self-assembling them into 3D supracrystals, we then focused on controlling the dimensions of our assemblies (Fig. 4). Specifically, by adjusting the ratio of the positively and negatively charged building blocks, N+/N-, we were able to halt the growth of the assemblies at points determined by the amount of excess NP of either type, ε = |N+-N-|. As described in our Nano Letters article (7, 1018, 2007), these excess nanoparticles formed protective/passivating, like-charged shells around the charge-balanced crystals, and thus stabilized the crystals of different sizes in solution. Fig. 4. Crystallization of equally sized, low-dispersity (s=15%) 5.2 nm Au(+) and 5.4 nm Ag(-) nanoparticles. (a) Average sizes of crystals grown with different amounts of excess gold or silver NPs (eAu/eAg, respectively). Dashed line is a theoretical fit to the expected d µ 1/eAg dependence. (b) The image on the left shows a vial containing a stable DMSO suspension of crystals grown at eAg = 0.1C. The graph has the distribution of crystal sizes determined by DLS (average size is 320 nm). Finally, in the beginning of the second year of the Award, we wrote an invited review for J. Phys. Chem. B (110, 2482, 2006) in which we provided a systematic overview of self-assembly in equilibrium and especially non-equilibrium systems. The non-equilibrium SA connected to our research on photoswitchable nanosystems, which we had originally proposed in our PRF grant application. While we had not managed to finalize this project within the two-year period of the Award, we have continued working on it and completed it this year. The exciting results of these studies (see Fig. 2, bottom) were described in this year Science (316, 261, 2007) and PNAS (104, 10305, 2007) papers. 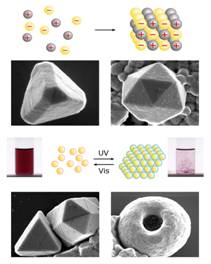 Year 1.
Year 1.