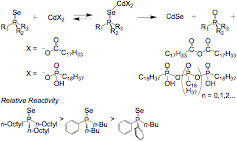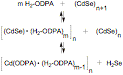ACS PRF | ACS
All e-Annual Reports

43069-AEF
Self-Assembled Binary Nano-Rod Arrays for Photovoltaic Materials
The synthesis of group II-VI colloidal nanocrystals by the thermal decomposition of organometallic precursors has had a major impact on nanoscience over the last 12 years. Despite the importance of this reaction, little has been done to study the reaction chemistry that converts cadmium and chalcogen precursor molecules to their II-VI materials. Using a combination of NMR (1H, 13C, and 31P), mass spectrometry, UV-Vis and fluorescence spectroscopy, we have investigated the synthesis of group II-VI semiconductor nanocrystals. These studies have shown that alkylphosphonic and alkylcarboxylic acid complexes of cadmium react with the trialkylphosphine chalcogenides in a deoxygenation reaction that cleaves the chalcogen atom, liberating a phosphonic or carboxylic acid anhydride, the corresponding trialkylphosphine oxide, and a soluble form of the cadmium–chalcogenide. Using NMR spectroscopy and the relative reactivity of aryl and alkyl phosphine selenides, we have provided evidence for a mechanism where alkylphosphonate and alkylcarboxylate ligands attack a Lewis acid activated (TOP=E)–Cd (E = S, Se, Te) complex, breaking the P=E double bond (Figure 1).
Figure SEQ Figure \* ARABIC 1
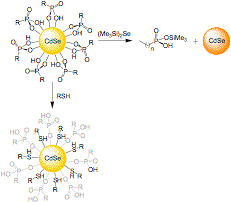
By measuring the kinetics of the precursor conversion reaction and crystal growth, we have shown that the formation of cadmium selenide from the precursors limits the rate of particle formation and can influence the number of particles formed during nucleation. These studies also suggest that traditional cadmium selenide syntheses, where particle growth is initiated by rapidly injecting CdMe2 with the phosphine selenide, have parallel reaction pathways that critically impact the initial rate of cadmium selenide generation. In particular, thermal decomposition of CdMe2 is competitive with protonation by the phosphonic acid surfactant that results in an initially rapid burst of cadmium selenide that depends on the kinetics of mixing (Scheme 1). Scheme SEQ Scheme \* ARABIC 1 My research has also provided evidence that the same phosphonic acids stabilize cadmium selenide solute molecules and thus impact the thermodynamics of particle nucleation. This hypothesis is supported by the observation that cadmium selenide dissolves in phosphonic acids solutions, and when in high concentration, can reversibly liberate hydrogen selenide. We showed that this dissolution also leads to nanoparticle ripening, the rate of which was shown to increase with added phosphonic acid. Finally the number of cadmium selenide particles formed during particle nucleation depends on the phosphonic acid concentration in a way that supports its role in stabilizing supersaturated solute molecules. These observations lead to a proposed equilibrium expression that describes the solubility of CdSe in our particle syntheses (Scheme 2). Scheme 2 The dependence of CdSe solubility on the phosphonic acid concentration led us to investigate whether phosphonic acids play a role in particle surface ligation. Previous investigations have concluded that CdSe nanoparticles are bound by trioctylphosphine oxide and trioctylphosphine selenide ligands. Using 1H- and 31P-NMR spectroscopy we were able to show that particle surfaces are bound by the octadecylphosphonic acid used in our particle synthesis. Monitoring attempts to displace the phosphonic acids with amines and thiols led to the surprising result that the added ligand rapidly binds to the particle surface without displacing the existing ODPA surfactant. Moreover, we found that ODPA could be cleaved from the surface by addition of (Me3Si)2Se or (Me3Si)2S, which caused the rapid (t1/2 < 1hr.) appearance of "free" ODPA molecules that was confirmed by their sharp 1H- and 31P-NMR spectra and electrospray ionization mass spectrometry (Figure 2). From these observations we conclude that a layer of cadmium octadecylphosphonate passivates the surface of CdSe nanoparticles where the ODPA molecules are bound by ionic interactions, rather than a simple dative interaction. Figure SEQ Figure \* ARABIC 2 These studies represent some of the first mechanistic and kinetic information on the conversion of precursor molecules to the semiconductor solute. They also provide some of the first evidence that phosphonic acid molecules are reagents in the precursor conversion in addition to stabilizing a solute form of the semiconductor. Finally, our study of cadmium selenide surface ligation significantly diverges from conventional knowledge of particle passivation and ligand exchange. These ideas help explain much of the irreproducibility that has plagued this area of science and lead to refined methods for nanoparticle crystallization as well as the growth of metal-chalcogenide shells. Our characterization of cadmium selenide surface reactivity will undoubtedly lead to better methods for ligand exchange and improve the implementation of these particles in technological applications. Career Directions My postdoctoral experience has helped me recognize that improved molecular understanding in nanoscience is greatly needed, and has given me the confidence to approach new problems in this area with the methods of a synthetic chemist. I plan to continue this interest as a professor at a Ph.D. granting institution. In particular I expect to use my skills as a small molecule chemist and a nanoscientist to develop and study water oxidation catalysts and solution processed silicon and germanium nanocrystals.