ACS PRF | ACS
All e-Annual Reports

42947-G5
Development of Robust Platforms for Membrane Proteins
Figure 1: Exploded view of the originally conceived five layer second generation device. |
Work on the microfluidic array device began with a brief redesign. As shown in Figure 1, the microfluidic device was originally planned to consist of five layers, with Glass, Si, PDMS, Si, Glass. Concerned about the effects of capacitance of the Si as well as some processing issues with the Si, we experimented successfully with glass-glass thermal bonding. We were able to obtain good bonds between top two and bottom two layers of the structure. Also problematic was the creation of the center layer. We molded the center layer of PDMS over patterned photoresist on a Si wafer, as is normally done. However, typically the PDMS is only bonded on the wafer side surface. For our device design, we wish to bond to both sides of the PDMS. We find that the exterior surface is significantly un-flat, as a result of the meniscus formed where the PDMS wet the photoresist post. This flatness caused the PDMS-glass bonding process to fail frequently, since the PDMS did not make good contact to the glass in the absence of applied pressure, or made good contact but strained in the presence of an applied bonding pressure. At this point we are attempting to solve these problems and test the device.
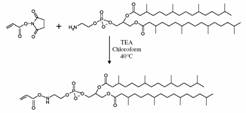
Figure 2: We created a cross-linkable lipid from N-acryloxysuccinimide and diphytanoylphosphatidylethanolamine. |
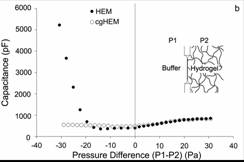
Figure 3: Capacitance (a proxy for membrane area) as a function of applied pressure for membranes bonded and unbonded to the gel on one side. The negative pressure data show the difference between the conjugated and unconjugated membranes. |