ACS PRF | ACS
All e-Annual Reports

42780-AC1
Palladium-Catalyzed Carbene Insertion into C-X Bonds
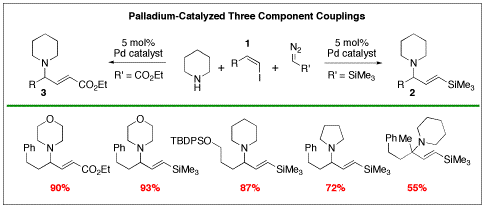
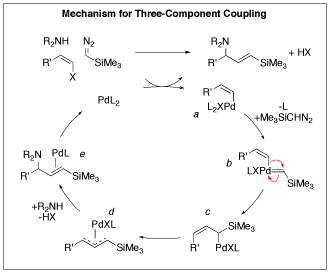
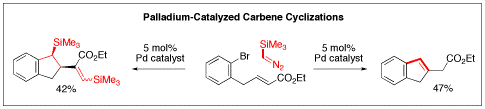
The precise assembly of organic molecules is a principal goal in modern organic synthesis. Our group is harnessing the ability of palladium catalysts to control the construction of molecules using carbenes, derived from diazo compounds, as one-carbon fragments. Addition of diazo compounds to palladium(II) complexes is known to generate palladium carbenes. We realized that the palladium(II) intermediates in many important catalytic reactions could capture carbene fragments and carry them into the final product. This reaction follows the important precedent of carbon monoxide insertion in palladium-catalyzed reactions. However, insertion of carbenes is more interesting than insertion of CO because carbene insertion generates stereogenic centers. We have made significant progress in two areas: three-component couplings to produce allylamines and carbene cyclizations to produce indanes. Three-Component Coupling to Allylamines We have developed efficient palladium-catalyzed three-component coupling reactions of vinyl halides, amines, and diazo compounds to produce allylamines in which the double bond is part of a vinylsilane or an enoate. Both types of functionality are important in organic synthesis. Vinylsilanes are important intermediates for stereospecific substitution reactions and enoates are important features of bioactive compounds and the synthetic routes used to make them. A number of students in my group have participated in this program. Initially Sean Devine developed the three-component coupling reaction using trimethylsilyldiazomethane, which is a convenient commercial reagent. The reaction generated a wide range of allylamines with a high degree of substitution. The reaction works best with cyclic six-membered ring amines. Acyclic primary amines like benzylamine gave much lower yields. Intramolecular versions of this reaction work to generate pyrrolidine rings. Xiao Jia has been developing this intramolecular reaction further. A mechanistic model that is consistent with our results and observations involves initial oxidative addition to form a vinylpalladium halide complex (a). Addition of the diazo compound to this electrophilic complex would generate a vinylpalladium carbene (b). Migration of the vinyl group to the carbene center would generate a pi-allylpalladium complex (c). Isomerization to the more stable eta-3-allylpalladium complex (d) precedes nucleophilic attack to give the E vinylsilanes (e). The stereochemistry is set in the migratory insertion step (b to c) and can not be lost through eta-1, eta-3, eta-1 interconversions. Indeed, in work undertaken by Will Heilbut, using the commercially available chiral ligand (R,R)-DIOP we obtained the product with 21% enantiomeric excess. Formation of chiral centers through palladium-catalyzed carbene insertions is an important advance in organic chemistry. We were delighted to find that the reaction also works with alpha-diazoesters like the commercial reagent ethyl diazoacetate. At present, the reaction requires a significant excess of ethyl diazoacetate relative to the vinyl halide component. Romas Kudirka is optimizing the reaction before we explore the scope with various vinyl halides. Carbene Cyclizations to Produce Indane Rings Romas Kudirka developed a palladium-catalyzed carbene insertion process to generate indanes, bicyclic structures with benzene rings fused to a five-membered ring. Indanes are present in a wide range of natural products like pterosins and bioactive compounds like the Alzheimer’s drug Aricept. Mechanistically the reaction proceeds via: 1) oxidative addition to produce an arylpalladium halide, 2) formation of a palladium carbene, 3) carbene insertion, 4) carbapalladation, 5) beta hydride elimination. By varying the conditions, we found that we could favor the insertion of either one or two carbene units. When one trimethylsilylmethylene subunit is inserted, the product undergoes protodesilylation under the conditions of the reaction; in these reactions the trimethylsilyldiazomethane serves as a methine precursor. Romas also showed that the tethered olefin could be replaced with an allene. Under these conditions secondary amines trap the resulting pi-allylpalladium complex. Since there are a variety of potential pathways we find that while the overall yield of indane products exceeds 80%, no single product is present in over 60% yield. Therefore we set this work aside to focus on the more productive three-component couplings to produce allylamines. Summary We are the first group to demonstrate that diazo compounds can participate in palladium-catalyzed carbene insertion reactions of aryl, benzyl, and now vinyl halides. Our work funded by the Petroleum Research Fund has allowed us to more fully demonstrate the power of palladium catalyzed carbene insertion reactions. The ability to precisely construct molecules, one carbon atom at a time, is truly on the cutting edge of organotransition metal catalysis.