ACS PRF | ACS
All e-Annual Reports

45026-AC5
Electron-Transfer Reactions of Transition Metal-Substituted 'Sandwich' Polyoxometalates on Electrode Surfaces
The development of electrocatalytic materials to replace Pt-based electrodes in low temperature fuel cells is an active area of current research. A promising strategy is the use of bimetallic catalysts, such as Co-Pd, that have been shown to exceed the performance of Pt. While the elementary steps of the oxygen reduction reaction (ORR) are not yet firmly established, thermodynamic analyses suggest that the ORR at a metal (M) electrode can be viewed as consisting of an initial dissociative chemisorption step (1) followed by the four-electron reduction of the oxide to water (2).
2M + O2 ==== 2MO (1)
2MO + 4e– + 4H+ ==== 2H2O + 2M (2)
Analysis of thermochemical data for these two reactions spanning a wide range of metals indicates that elements such as W, which form stable bonds to O, perform well for O2 bond scission and poorly for O atom reduction. Conversely, metals such as Au, which reduce adsorbed O atoms efficiently, are ineffective with respect to O2 bond scission. Based on these observations, a simple guideline for the development of bimetallic catalysts has been proposed: combine a metal that is good at O-O bond scission with a second metal that reduces adsorbed O atoms efficiently. A possible difficulty with this approach is that, in the case of metals that form stable M-O bonds, there would be a tendency for the O atoms generated in reaction 1 to remain adsorbed on the first metal, effectively shutting down reaction 2. In practice, a compromise is generally sought, as in the case of bimetallic catalysts such as Co-Pd, where one metal is slightly better at bond scission (Co) and the second is slightly better at reducing adsorbed O atoms (Pd).
Polyoxometalates (POMs) are nanometer sized metal oxygen containing anions that have a wide range of catalytic applications. We believe that transition metal substituted POMs adsorbed on the surface of an electrode that is catalytically active for the reduction of adsorbed O atoms (i.e., Au, Pd, Pt) should function in much the same way as a typical bimetallic ORR catalyst. In this contribution, we set out to design POM co-catalysts for Au, Pd and Pt electrodes.
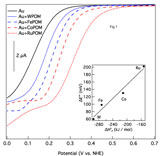
Representative linear sweep voltammograms (scan rate, 10 mV/sec) showing the influence of a series of P2W17MnO62(12-n)– POMs on the electrochemical reduction of dioxygen at an Au electrode immersed in 0.10 M HClO4 are presented in Figure 1. Au was chosen as the cathode for these measurements because it is not catalytically active for O2 bond scission as evidenced by an unfavorable free energy change for reaction 1. To avoid visual clutter, only the reductive sweeps are shown. The solid black curve shows the ORR behavior of a clean Au electrode in the absence of added POM, and is consistent with previous research on this system. As indicated by the dashed curves, addition of POM to the supporting electrolyte leads to a significant and reproducible positive shift (ÆE°') in the O2 reduction potential. The concentration of POM added to the supporting electrolyte was optimized separately for each compound to give the maximum shift of the ORR potential. ÆE°' increases in the order W < Fe < Co < Ru, which correlates extremely well with the heats of formation (ÆH°f) of the corresponding bulk metal oxides (inset to Fig. 1). This behavior is in agreement with the predictions of the simple thermodynamic models discussed earlier, namely, that POMs with stronger M-O bonds exhibit a weaker influence on oxygen reduction at Au surfaces (i.e., a less positive shift of the potential) than POMs with weaker M-O bonds.
Figure 2 shows the response of Pt electrodes immersed in electrolytes containing a range of concentrations of a Co-substituted Keggin POM. In contrast to the behavior of Au electrodes, on Pt, both positive and negative shifts are seen, depending on the concentration of POM added to the electroyte. Negative shifts of the O2 reduction potential can be rationalized on the basis of site blocking effects that influence the kinetics of the ORR on Pd and Pt electrodes (e.g., by inhibiting the formation of the metal oxide). The dramatic positive shift of ca. 55 mV observed for 20 µM Co-Keggin is similar to what has been reported using bimetallic Pt-based nanoparticle catalysts. In conclusion, transition metal substituted POMs adsorbed on the surface of oxygen reduction cathodes (such as Au) lead to positive shifts of the ORR potential by a mechanism similar to that which has been proposed for bimetallic ORR catalysts. The magnitude of the shift can be quite large (ca. 200 mV), is transition metal dependent, and can be explained using simple thermodynamic concepts. In the case of more active cathode materials such as Pt, our results indicate that there is an optimum concentration of POM, where catalytic activity and site-blocking effects are in balance.