
ACS PRF | ACS
All e-Annual Reports

44181-AC10
Self-Assembly of DNA-Colloidal Systems: from Interactions to Structure
The focus of this theoretical project are micro- and nanoparticles with DNA-mediated interactions. In a typical experimental scheme, submicron colloidal particles are functionalized with certain single-stranded DNA chains (ssDNA). By changing the concentration of "linkers" (the DNA molecules whose ends are complementary to the chains attached to particles A and B, respectively) one can switch "on" and "off" the attractive force acting selectively between particles of type A and B only.
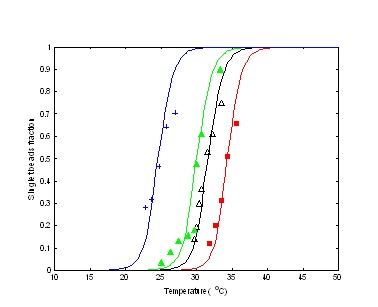
Study of random aggregation and melting. The aggregation in DNA-colloidal systems observed in a number of experiments carries important information about the underlying interparticle interactions. Within this project, a statistical mechanical approach was used to relate the macroscopic behavior with the microscopic properties of the system, such as DNA coverage and hybridization free energy. The calculated aggregation/melting curves are in a very good agreement with experiments done by two different groups with two very different types of particles. What is especially encouraging is that both the position and the width of the melting curves were correctly fitted with a single model parameter.
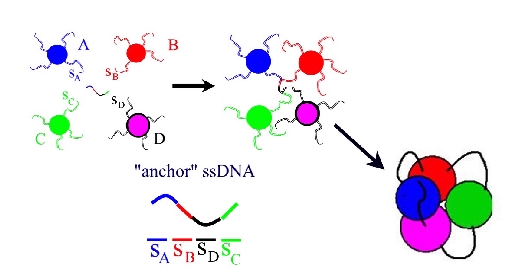
Programmable self-assembly of micro- and nanoparticle clusters. As an alternative to the traditional bulk self-assembly, we proposed to build relatively small particle clusters of prescribed geometry. These clusters can be subsequently used in hierarchical self-assembly schemes as building bocks for larger structures. The particular scheme for the cluster self-assembly is represented in Figure below. There is a number of potential problems in the proposed scheme. First, the cluster consisting of exactly one DNA- anchor and n particles should be the dominant multiparticle structure in the solution. In true thermal equilibrium, this turned out to be an extremely hard condition to satisfy. Fortunately, it is much easier to achieve regime when these clusters are the dominant metastable (but long-living) structures. Another potential problem is the possibility of multiple competing shapes for the same cluster. It was found that the clusters "fold" directly into their ground state configuration, given that the DNA linkers are flexible enough and their gyration radii are of the order of the particle size. Furthermore, the shape of the cluster can be "programmed" by additional interparticle links within the cluster.
What makes "hairy particles" slow? In order to facilitate the self-assembly of ordered DNA-colloidal structures one has to understand the origin of the experimentally observed slow relaxation dynamics in these systems. Our study reveals a number of peculiar properties of this model system. First, there is a wide power-law-like distribution of the relaxation times which is indeed consistent with experimental data. Second, the lateral particle motion is typically an anomalous diffusion. . Finally, the system exhibits "aging" effect: as the particle explores the binding energy landscape by diffusion, it is able to find dipper and dipper minima and hence dramatically extend the bond lifetime. From the practical point of view, an important prediction of our theory is that the fastest dynamics is achieved when a large number of weak bonds are used instead of small number of strong ones. Note that most of the experiments to date were done well outside of this optimal regime.
Cooperative key-lock binding and drug delivery. The above model is not limited to particle with DNA-mediated interactions. Very similar ideas can be applied to other systems with key-lock binding, which may be of a great biological and medical importance. In particular, we have studied the properties of dendrimer particles decorated with functional groups capable of selective adsorption to certain membrane proteins in living cells. These nanodevices have been suggested for cell specific drug delivery, e.g. for chemotherapy. The scheme is based on the notion that certain membrane proteins (e. g. folic acid receptors) are more expressed in cancerous cells than in normal ones. This contrast should be further enhanced due to the cooperative binding: if each nanodevice can make more than one bond to target the proteins, its binding will be highly specific to cancerous cells. However, our analysis of in-vitro experiments shows that the cooperativity is kinetically limited. The non-trivial prediction of our theory is that the cooperativity and hence the specificity can be enhanced by reducing the strength of individual key-lock bonds. We have suggested a particular realization of this scenario by using the DNA-mediated scheme.
PRF funding was essential in identifying the main directions for the future research of PI's group, as well as in initiating the scientific carrier of the supported graduate student, Nick Licata.