ACS PRF | ACS
All e-Annual Reports

43447-AC7
Block Copolymers for the Formation of Mono- and Multi-Functional Metallic Nanoparticles
Metallic particles with nanometer-scale dimensions are of general interest as well-defined building blocks in nanotechnological applications and of specific interest for the size-, shape-, and metal-specific properties that they exhibit. Control over the extent of surface functionality on these nanoparticles is a goal of fundamental importance in the further development of nanoscale materials. As surface ligands dictate the manner in which nanoparticles interact with their chemical surroundings, development of reliable routes to nanoparticles with specific numbers of functional groups will enable control over their incorporation into larger assemblies.
The primary aim of the research project is to demonstrate the preparation of nanoparticles with controlled surface functionality. We have found that low temperature thermolysis (100–200 °C) of cobalt carbonyl adducts of alkyne-functional polymers results in the formation of cobalt nanoparticles with concomitant cross-linking of the alkyne-functional blocks.
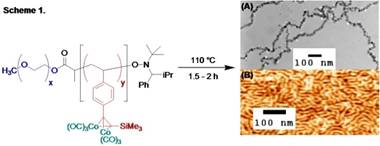
During the period of the grant, we have begun to study the thermal cross-linking reactions of cobalt carbonyl-modified diblock copolymers for the preparation of cobalt nanoparticles. Block copolymers have been prepared by a range of controlled free radical polymerization techniques from appropriately functionalized poly(ethylene oxide) macroinitiators. For example, reaction of poly(ethylene oxide)-block-poly(trimethylsilylethynylstyrene), prepared by atom transfer radical polymerization, with cobalt carbonyl affords a cobalt-functional polymer that affords cobalt nanoparticles upon heating (Scheme 1A). The tendency of the particles to form one-dimensional strands suggests that they are magnetic and further characterization of the magnetic properties of these particles is underway. Similar copolymers with larger blocks lead to the formation of cylindrical nanostructures after heating (Scheme 1B).
Further research efforts will be needed to better understanding the nanoparticle formation process through variation of polymer size and composition and through more detailed analysis of the elemental composition of the formed particles. We will also continue to investigate the formation of smaller particles with controlled levels of surface functionality by conducting thermolysis studies at high dilution. The chemical structure of the cross-linked shell will be characterized by spectroscopic techniques including IR, Raman, and NMR spectroscopy, though the magnetic properties of cobalt nanoparticles are likely to minimize the usefulness of standard NMR techniques, unless the magnetic cobalt can be removed without significantly altering the structure of the polymer shell. The awarded funds have supported two graduate students, Dr. Laura Sessions and Mr. Addison Bouchard, and have been used to purchase supplies for this work and to support presentations by both graduate students at meetings of the American Chemical Society in 2006 and 2007. An undergraduate student, Jeffrey Garber, has participated in this research and will present a poster about his work at the Spring 2008 meeting of the American Chemical Society.