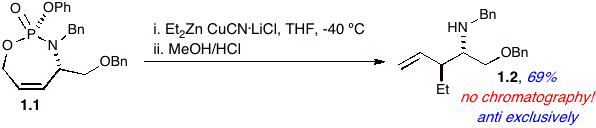ACS PRF | ACS
All e-Annual Reports

42457-AC1
New Phosphorus and Sulfur Heterocycles for Synthesis
Divalent Activation Imparted by Phosphoramidates
In expanding our understanding and utilization of phosphate tethers [1] in facilitating synthetic applications, non-racemic phosphoramidate 1.1 was formed (Scheme 1). We were pleased to observe that when 1.1 is exposed to a Et2Zn-derived cuprate, the reaction proceeds with complete selectivity to displace the allylic phosphate moiety. An underlying principle of this approach utilizes the well-documented superiority of phosphates as leaving groups in anti-SN2' displacement reactions, first reported by Yamamoto and coworkers [2]. This feature, in which the stereochemistry of an allylic phosphate is inverted in an anti-SN2' displacement, has been successfully employed in previous strategies that exploit high stereospecificity for chirality transfer [3]. As expected from our earlier experiences with temporary phosphorus tethers, the P-N bond is cleaved and upon NaOH work up the di-substituted homoallyl amine 1.2 is isolated as a single diastereomer in excellent purity without the use of column chromatography.
Scheme 1
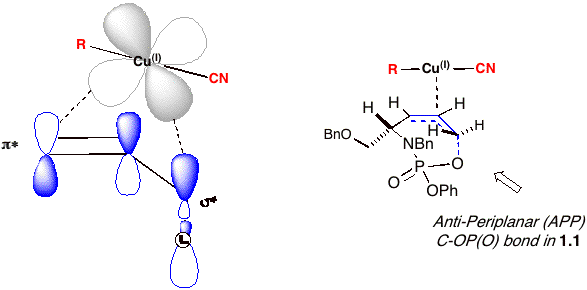
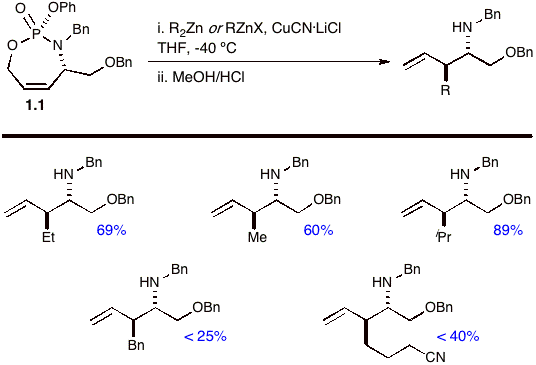
The remarkable selectivity for this transformation can be rationalized using Corey's proposed concerted, asynchronous mechanism [4] for cuprate additions as highlighted in Figure 1. A prerequisite for the reaction requires the leaving group to be orthogonal to the p system, i.e. co-planar alignment of the s* and p* orbitals. In this mechanism, Corey surmises that the reacting cuprate simultaneously coordinates both the p* orbital of the olefin and s* orbital of the phosphate ester leaving group. The asynchronous nature of the transformation predicts a TS in which substantial bond-lengthening occurs with respect to the s* bonding orbital. In phosphoramidate 1.1, the most electrophilic s* orbital originates from the C-O of the allylic phosphate (which correlates to its LG ability), and thus the reaction proceeds exclusively through the only accessible s* and p* orbital aligned conformer to yield anti product 1.2. Figure 1 The Corey Mechanism in Phosphoramidate 1.1 Additional reactions of 1.1 with an array of zinc-based organocuprates gave good to excellent yields and selectivities when using 4-5 equivalents of dialkylzinc-derived organocuprates (Scheme 2). Treatment of 1.1 with 8-9 equivalents of the alkylhalozinc derived organocuprates [R = Bn and (CH2)3CN, Scheme 2] provided poorer yields of the disubstituted homoallyl amines. Scheme 2 We next focused on the readily prepared non-racemic phosphoramidate 3.1, where the chiral center is located on the allylic carbinol. We were pleased to observe that cuprate additions produce chiral b-substituted homoallyl amines 3.2 in excellent yield and enantioselectivity (>97% ee via Mosher amide). As with 1.1, excellent purities were realized by this procedure without column chromatography. Scheme 3 Literature Cited: [1] Whitehead, A.; McParland, J. P.; Hanson, P. R. Org. Lett. 2006, 8, 5025-5028. [2] Yanagisawa, A.; Noritake, Y.; Nomura, N.; Yamamoto, H. Syn. Lett. 1991, 4, 251-253. [3] (a) Belelie, J.L.; Chong, M. J. Org. Chem. 2001, 66, 5552-5555. (b) Calaza, M.I.; Hupe, E.; Knochel, P. Org. Lett. 2003, 5, 1059-1061 and references therein. [4] Corey, E. J.; Boaz, N. W. Tetrahedron Lett. 1984, 25, 3063-3066.