ACS PRF | ACS
All e-Annual Reports

45518-G4
Two-Dimensional Fourier Transform Spectroscopy of Resonance Energy Transfer in Biological Molecules
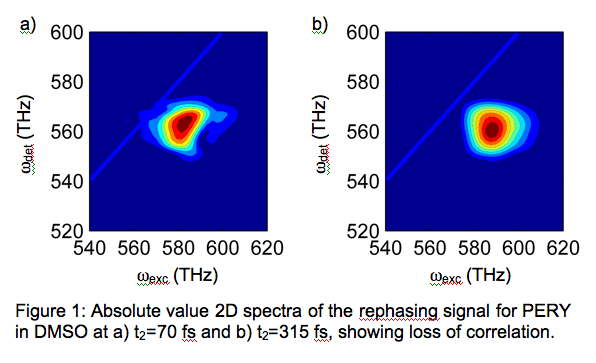
Our primary goal is to understand how biological molecules harness energy and efficiently convert it from one form to another to drive biological functions. We have proposed to use two-dimensional electronic spectroscopy (2DES) in the visible wavelength regime to study several natural and artificial light-harvesting systems. Although energy transfer processes in photosynthesis have been studied extensively with nonlinear optical spectroscopy, the electronic couplings governing the energy transfer are usually inferred indirectly. Most nonlinear spectroscopies are inherently one-dimensional, revealing broad features that mask individual transitions and their couplings. By spreading the linear spectrum into two dimensions, 2DES exposes the connectivity of the light-harvesting system, allowing energy transfer to be followed on a chromophore-by-chromophore basis, with femtosecond time resolution. 2DES promises the most complete picture to date of energy transfer processes: traditional nonlinear spectroscopies are a subset of this measurement. In the past funding year we have met two primary goals towards applying 2DES to the study of light-harvesting systems: 1) we have completed our two-color 2DES setup, 2) obtained several natural light-harvesting systems for study. In addition we have begun modeling our preliminary data on a simple dye system. 2DES is the optical analog of the simplest multidimensional NMR experiment. In 2DES, an optical “pump” pulse excites the sample, setting its charges in motion at their natural oscillation frequency. Depending on what this frequency is, a second “pump” pulse, arriving a delay t1 later, may enhance or suppress the initial oscillation. After a second delay t2, during which the sample can relax, a third “probe” pulse excites the sample and the emitted “echo” is recorded as a function of time t3. Fourier transforming with respect to the t1 and t3 variables yields a 2D spectrum at each time delay t2. We have built two noncollinear optical parametric amplifiers (NOPAs) to provide tunable visible light over a broad bandwidth from ~475 nm - 800 nm, allowing us to study coupled electronic transitions within this region. We generate the excitation pulse sequence for the experiment by sending the pump and probe beams into a passively phase-stabilized diffractive optics (DO) arrangement, as in our previous work. The two-color 2DES apparatus that we have built is sufficient for our proposed studies of light-harvesting complexes. To optimize our 2DES apparatus we have made preliminary measurements in N,N-bisdimethylphenyl-2,4,6,8-perylenetetracarbonyl diamide (PERY) in dimethyl sulfoxide (DMSO) solution. Figure 1a,b show representative 2D spectra at different t2 values. The acquisition time was ~3 seconds, under low excitation conditions (~5% bleach). At early t2 the data is elongated along the diagonal, reflecting inhomogeneous broadening. At later times the diagonal elongation vanishes as the system loses memory of its initial excitation frequency, consistent with 2D measurements of similar dye systems. This data is preliminary and is meant to demonstrate the general quality of the data we can obtain within seconds with no signal averaging. Descriptions of the quantum dynamics of condensed phase systems as complex as light-harvesting complexes remain an unmet challenge. Despite increases in computing power, exact quantum treatments of these systems are impossible. Working in close collaboration with Eitan Geva we are employing a powerful formalism for calculating 2D spectra based on the nonlinear response function. Comparison between the calculated and experimental spectra will allow the set of local coordinates, their couplings, and the description of how they interact with the surrounding bath to be refined. We are currently modeling our PERY data, explicitly accounting for coupling between known intramolecular vibrations and electronic transitions. Once satisfactory agreement is achieved in this simple system we will publish this work and extend the modeling to more complex natural light-harvesting systems. In the past year we have worked in collaboration with groups in the Life Sciences Institute and the Department of Molecular, Cellular and Developmental Biology at the University of Michigan to isolate two natural photosynthetic complexes of interest. We have expressed and purified DNA Photolyase, a simple DNA repair protein and have also established a collaboration with Charles Yocum to examine the reaction center complexes from Photosystem II from spinach.