
ACS PRF | ACS
All e-Annual Reports

41962-AC6
Spectroscopic Investigation of Zwitterion Formation in Hydrated Amino Acid Clusters
PRF support enabled our group to extend its efforts involving the structural characterization of reaction intermediates in water clusters to include a systematic study of binary proton-bound complexes of relatively large alcohols, ethers, and amines. The motivation for this extension arose from initial work directed at studying zwitterion formation in the micro-hydrated aminoacids, a process that involves transport of a proton along a “water-wire”. This transport mechanism is ubiquitous in biological systems such as trans-membrane proton pumps, but even more importantly in the context of PRF-supported 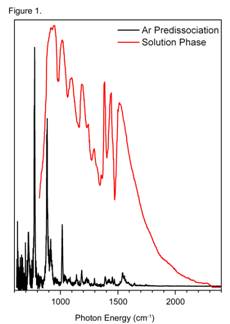 research, lies at the heart of proton transport essential for the operation of fuel cells.
research, lies at the heart of proton transport essential for the operation of fuel cells.
The key issue in transport regards the nature of the binary, intermolecular proton bond, as this condition represents the static state of the system between proton hops, and the potential energy profile for proton translation between the heavy atoms determines the probability of a thermally activated hop. A prototypical example of a binary complex is the proton-bound dimer of diethyl ether, (CH3CH2)2O {H+} O(CH2CH3)2. Of course, such compounds have been studied for decades either stabilized in solution or trapped in cryogenic salt crystals.
Our approach exploits recent advances in gas-phase ion chemistry, where we use Ar-mediated synthesis to generate cold complexes isolated in a mass spectrometer:
H3O+·Arn + R2O → R2O{H+}H2O·Arm + (n-m) Ar
R2O{H+}H2O·Arm + R2O → R2O{H+}OR2·Ar + H2O + (m-1) Ar.
The Ar “tagged” product is of primary importance, as this species can be studied using infrared action spectroscopy:
R2O{H+}OR2·Ar + IR → R2O{H+}OR2 + Ar
in a linear absorption regime. The advantages of this approach are dramatically illustrated by the comparison between the solution-phase spectrum of the proton-bound diethyl complex and that obtained using the Ar-tagging method in Fig. 1. Before the Ar-predissociation results, the prevailing view of these systems was that the diffuse infrared bands such as those shown in Fig. 1 for the Et2O{H+}OEt2 were an intrinsic property of these complexes, and further that this band structure arose from extensive mixing between the light coupling proton and the vibrational modes of the flanking molecular constituents. The observation of a sharp, simple spectrum using Ar-predissociation thus drastically changes this picture to one where the shared proton vibrations are distinct, with sharp level structure that dominates the infrared fundamentals arising from the molecular components.
Because the Ar-tagged spectra reveal the intrinsic vibrational level structure of the shared proton, we were able to carry out a systematic study of how its vibrational eigenstates depend on the chemical properties of the molecules to which it is bound. This, in turn, directly reflects the shape of the potential energy surface that effectively traps the shared proton. A survey of over 20 complexes yielded the results summarized in Fig. 2, where we plot the dependence of the shared proton vibrational frequency against the difference between the thermodynamic proton affinities of the separate molecules that are bound to it. Note the remarkably strong correlation, revealing a universal dependence that should prove to be of general value in evaluating the proton transport barriers in a variety of systems.
 |